Zika Virus Unveils Its Stealthy Tactics: Building Nanotubes to Cross the Placenta
2025-03-18
Author: John Tan
In 2015, the emergence of the Zika virus triggered a significant health crisis across the Americas, causing many to question how this seemingly benign virus could lead to devastating birth defects when contracted by pregnant women. Researchers have now uncovered a chilling mechanism by which the virus can stealthily cross the placental barrier, raising alarms about its potential future impacts.
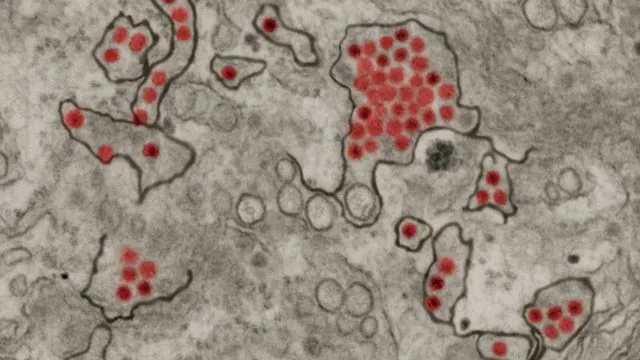
A collaborative study by researchers from Penn State and Baylor College of Medicine has revealed that the Zika virus constructs minute structures known as tunneling nanotubes. These tiny tunnels allow the virus to facilitate the transfer of viral material needed to infect adjacent cells, including those within the placenta. This discovery is pivotal as it explains how Zika can navigate from mother to child without provoking the immune system’s defenses.
Published in Nature Communications, the research identified a particular Zika virus protein—non-structural protein 1 (NS1)—as essential for the formation of these nanotubes. This breakthrough not only opens doors for understanding the virus's cunning behaviors but also sets the stage for developing preventive measures and potential antiviral therapies. The research was bolstered by nearly $4 million in funding from the U.S. National Institute of Allergy and Infectious Diseases, received in 2024.
“Zika must breach the placental barrier to infect newborns,” explained Anoop Narayanan, a research professor at Penn State and lead author of the study. “This discovery could be crucial in finding ways to halt the virus’s transmission from mother to fetus.”
Zika is part of the Orthoflavivirus genus of the Flaviviridae family, which includes notorious pathogens such as West Nile virus, dengue virus, and yellow fever virus. While typically transmitted through mosquito bites, Zika has the unique ability to spread from person to person without a vector and is the only virus in its family known to cross the placental barrier.
While Zika infections in adults usually present mild symptoms, the consequences for unborn children can be life-altering, leading to conditions such as microcephaly and other neurological disorders. Presently, there is no vaccine or specific antiviral treatment available for Zika, underscoring the urgency of this research.
“Finding a preventive solution for this infection is vital,” asserted Joyce Jose, an associate professor at Penn State and co-author of the study. Even with a decline in reported Zika cases, Jose warns that changes in climate could facilitate the spread of Zika-bearing mosquitoes into new regions, potentially sparking future outbreaks.
The team serendipitously discovered Zika’s ability to form these nanotubes while observing infected live cells under fluorescent microscopy. They noticed that the Zika virus induced distinct tube-like structures that were absent in cells infected with other viruses, such as dengue and yellow fever. This unique observation led to a deeper investigation, revealing the prominence of these nanotubes especially in placental cells.
Interestingly, similar mechanisms for cell-to-cell transfer are observed in other viruses like HIV, herpes, and SARS-CoV-2, the virus responsible for COVID-19; however, these viruses do not cross the placenta. In controlled laboratory conditions, the researchers found that Zika-infected placental cells utilized these nanotubes to transfer viral particles and even cellular components—like mitochondria—between cells, thus enhancing viral growth and spreading abilities.
“It’s a two-way street,” Narayanan pointed out. “The virus reprograms the entire cell environment to ensure its survival and proliferation.”
The NS1 protein stands out in this dynamic process, as it is the driving force behind the construction of the nanotubes specific to Zika. Understanding the exact area of NS1 responsible for this formation was a crucial step in the investigation, with pivotal contributions from Shay Toner, a doctoral student at MIT, who focused on this aspect during his time at Penn State.
“Being involved in such impactful research as an undergraduate was an incredible opportunity,” Toner reflected.
The team’s future endeavors will focus on pinpointing the specific signaling pathways activated by NS1, aiming to identify new targets for antiviral drugs. Additionally, they have plans to conduct further studies using mouse models.
“As we delve deeper into this research, we find ourselves piecing together a complex puzzle,” Jose noted. “There are many more questions to answer as we explore the formation of these nanotubes and their role in Zika transmission.”
This groundbreaking research not only enhances our understanding of the Zika virus but also highlights the ongoing threat it poses to pregnant women and infants, making it a critical focus for public health efforts in the years to come.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)