WHO Launches Groundbreaking Scheme for Malaria Diagnostic Quality: Revealing Key Insights from Six Years of Evaluation
2025-03-23
Author: John Tan
Malaria continues to be a formidable global health challenge, with a staggering 608,000 fatalities wrought by the disease in 2022 alone. The vast majority of these deaths occurred in sub-Saharan Africa, highlighting the pressing need for effective diagnostic methods in malaria-endemic regions. Since 2010, the World Health Organization (WHO) has emphasized the necessity of confirming malaria infections through parasite-based diagnostics prior to treatment initiation.
Historically, malaria diagnostics predominantly relied on microscopy and rapid diagnostic tests (RDTs) that detect specific antigens. However, in recent decades, the landscape has transformed with the advent of nucleic acid amplification tests (NAATs), offering significantly enhanced sensitivity and specificity for identifying Plasmodium infections. Initially developed in the early '90s, these methods have advanced with modalities such as real-time PCR, nested PCR, and CRISPR-based detection techniques.
NAATs are not only crucial in clinical trials for new anti-malarial drugs and vaccines but also play a pivotal role in epidemiological research, particularly in low-transmission settings where conventional methods might fall short. Their ability to detect as few as 1 to 100 parasites per milliliter of blood makes them indispensable in the quest for malaria elimination in regions previously deemed free of high transmission levels.
Recognizing the need for consistent and reliable diagnostic capabilities, WHO, in collaboration with the UK National External Quality Assessment Service (NEQAS), initiated the Malaria NAAT External Quality Assessment (EQA) scheme in January 2017. This program targets both high and low transmission settings, promoting laboratory performance and harmonization in testing methodologies. Over the course of six years, this scheme has facilitated the distribution of Plasmodium sample panels to participating laboratories bi-annually, allowing them to evaluate their diagnostic accuracy against international standards.
Analysis of the first three distributions revealed that various factors—including the laboratory type and sample technology—significantly influenced diagnostic performance. With additional data accrued through subsequent distributions, a more comprehensive understanding of the changes in laboratory performance has emerged. The latest report spans eleven distributions and uncovers various challenges faced by participating laboratories, reinforcing the importance of reliable quality controls in malaria diagnostics.
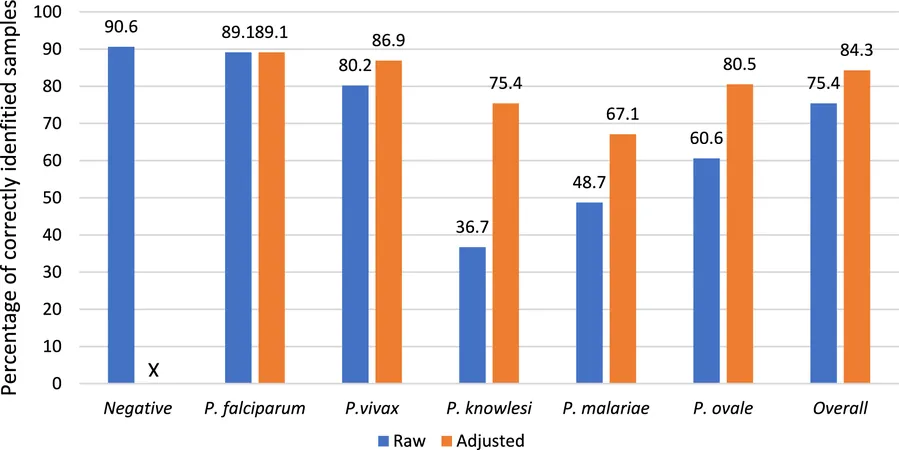
As of now, 75 laboratories across 42 countries have embraced the EQA scheme, collectively submitting results that underscore the gradual enhancement of diagnostic capabilities over time. Notably, accuracy rates for identifying negative samples soared to around 90.6%, while P. falciparum—which accounts for the majority of malaria cases—was correctly identified in roughly 89.1% of positive samples.
Despite these successes, discrepancies remain: while performance improved significantly for detecting common species like P. falciparum and P. vivax, identification rates for rarer forms such as P. knowlesi and P. malariae lagged, suggesting an urgent need for enhanced training and resources in certain laboratories. The reasons behind inconsistencies in laboratory participation ranged from logistical problems related to sample imports to funding issues exacerbated by the COVID-19 pandemic.
Looking toward the future, the scheme is set to introduce new assessments targeting anti-malarial drug resistance markers, as well as monitoring genetic deletions known to hinder detection by standard tests. By continually adapting and expanding the scope of the EQA scheme to include emerging threats, WHO aims not only to bolster malaria diagnostics but also to contribute crucial information in the global fight against infectious diseases.
As the demand for advanced malaria diagnostics continues to evolve, this EQA initiative reflects an unwavering commitment to enhance diagnostic accuracy, ultimately saving lives and improving health outcomes in malaria-stricken regions.
In conclusion, participating laboratories are encouraged to incorporate the strategic feedback provided by the EQA scheme to refine their methods further. By fostering a collaborative environment among laboratories, WHO envisions a future where rapid advancements in NAAT proficiency can directly translate into improved mortality and morbidity stats in the ongoing battle against malaria.



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)