Unveiling the Secrets of STING: Why Human Cancer Therapies Need a Fresh Approach
2025-07-03
Author: Sarah
Scientists have turned the spotlight on the STING (Stimulator of Interferon Genes) pathway, a crucial player in harnessing the immune system to battle cancer. This pathway bolsters the body’s defenses against harmful pathogens and has immense potential to activate an innate immune response specifically targeting cancer cells. However, groundbreaking research in the journal *Nature Chemical Biology* is shaking up conventional wisdom.
For years, the focus has been on *activating* STING to recruit immune cells that wage war on tumors. Yet, a new study reveals the importance of *inhibition*, indicating that overstimulation of STING may lead the immune system to mistakenly attack healthy cells. This nuanced understanding positions the STING pathway as both a powerful ally and a complex adversary in drug development—particularly for human therapies.
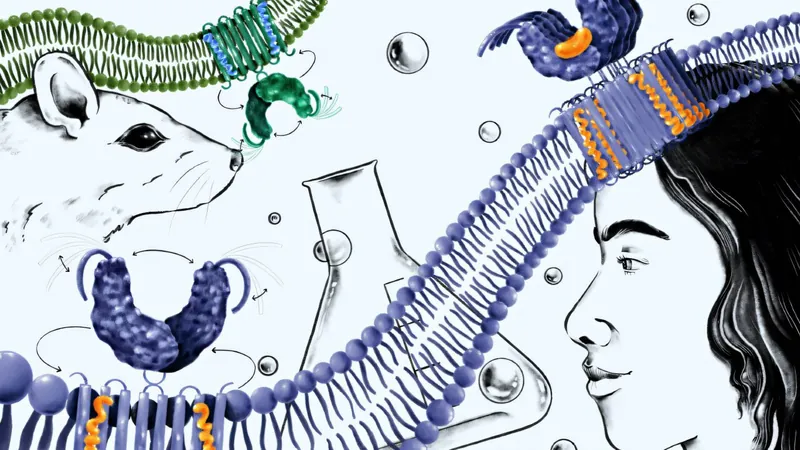
Led by biochemist Lingyin Li, this transformative study evaluated *H-151*, the most advanced STING inhibitor designed for humans. While it showed success in reversing cognitive decline in mice, it surprisingly failed to suppress STING signaling in purified human blood cells. "What we uncovered was startling: in humans, the binding site for H-151 is missing a key pocket found in mice, making drug customization a significant hurdle," explains Li, a distinguished professor at Stanford University.
H-151's strength lies in its ability to tightly bind to STING, yet its effectiveness varies dramatically between species, underscoring the limitations of using mouse models to predict human results. To address this challenge, Li's team meticulously analyzed the necessary steps in human STING signaling and discovered that oligomerization—the assembly of STING molecules—is crucial for triggering immune responses.
Inspired by STING’s built-in auto-inhibitory mechanism, the team developed a novel proof-of-concept molecule designed to block oligomerization, thus halting STING from forming the large complexes essential for immune activation in humans.
Xujun Cao, a leading author of the study, stressed, "Our findings highlight the urgency of creating STING inhibitors tailored specifically for humans. The identification of this distinct target site provides a roadmap for others aiming to find universal targets that can mitigate STING autoimmunity."
Rebecca Chan, another first author, added, "The precision required for STING to activate is fascinating. If the activation process were too easy, our immune systems might inadvertently turn against our own cells. This meticulous mechanism is actually beneficial."
What’s next? The Li lab is set to explore whether their insights into STING inhibition could open new doors in treating not just cancer but also neurodegenerative and autoimmune diseases. As they pave the way for innovative molecular candidates, the quest for human-ready STING inhibitors for clinical applications is well underway.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)