Unveiling the Secrets of Severe COVID-19: How Demographics, Health Factors, and Viral Genomics Interact Using Machine Learning
2025-01-28
Author: Sarah
The COVID-19 pandemic has wreaked havoc worldwide, with estimates revealing around 700 million cases and approximately 7 million fatalities up to March 2024. The clinical presentation of the disease varies dramatically, from asymptomatic cases to critical illness and death. In fact, approximately 80% of infections are mild to moderate, while about 19% progress to severe or critical conditions when looking at the pre-vaccination data. The World Health Organization (WHO) categorizes a respiratory failure or oxygen saturation below 94% as severe COVID-19, leading to an increased demand for healthcare resources.
Ongoing research has solidified our understanding of how certain host factors — such as age, gender, and existing health conditions — heighten the risk of developing severe disease. A systematic review indicates that older patients, males, those with underlying health issues like diabetes or heart disease, or individuals who are immunocompromised are more likely to experience devastating effects from the virus.
However, socioeconomic factors also play a crucial role in determining who faces the greatest risks. Studies indicate that racial minorities, essential workers, and those with inadequate access to healthcare suffer disproportionately from COVID-19, revealing the intersection of health and systemic inequalities.
Meanwhile, the ever-evolving SARS-CoV-2 virus has generated numerous variants, each bringing unique challenges to public health systems. Variants of concern (VOCs), including Alpha, Delta, and Omicron, have demonstrated variances in transmissibility, clinical severity, and vaccine resistance. The relationship between viral genomic factors and individual patient characteristics is complex. For example, the Alpha variant has been linked to higher disease severity, while the Omicron strain is associated with milder symptoms.
In light of these complex interactions, our study sought to explore how patient demographics and SARS-CoV-2 genomic data can be integrated using machine learning to better predict the severity of COVID-19. Drawing from a comprehensive dataset of COVID-19 patients across 11 hospitals in the Greater Toronto Area, we analyzed clinical factors such as age, sex, and existing health conditions combined with detailed genome sequencing of the virus.
We trained machine learning models on the linked datasets to identify key predictors of hospitalization. Our findings revealed that clinical features like pre-existing vascular and pulmonary diseases, along with fever, emerged as strong indicators of severe illness. Interestingly, genomic factors contributed less than anticipated, with the most notable signatures arising from pre-VOC variants.
This comprehensive analysis highlights an intrinsic challenge faced by researchers: while demographic and clinical factors significantly inform hospitalization rates, the emerging viral genome is less impactful. As a crucial part of understanding the pandemic's evolution, this study underscores the importance of leveraging machine learning to dissect complex relationships — and potentially predict severe outcomes in future viral outbreaks.
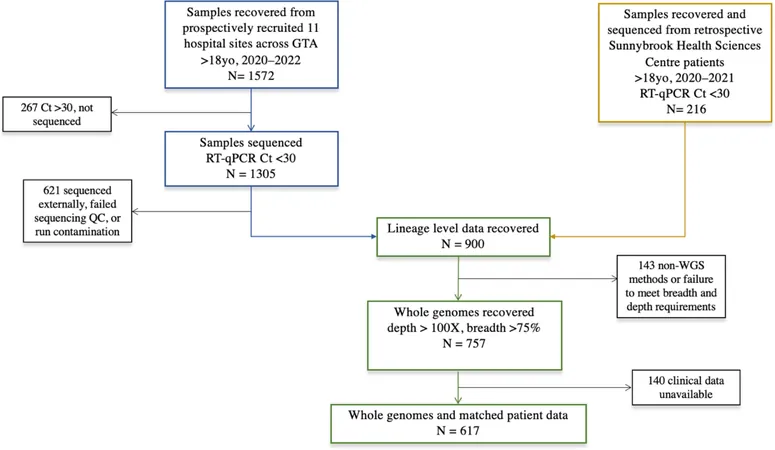
From March 2020 to April 2022, 1,572 COVID-19 samples were collected, leading to a focused cohort of 617 patients with complete clinical and genomic data. Notably, inpatients had a mean age significantly higher than that of outpatients, with pre-existing comorbidities being more prevalent among hospitalized individuals. Symptoms varied greatly, with cough and fever being the most reported.
The research leverage machine learning frameworks effectively, employing varied algorithms to predict hospitalization status based on demographic and genomic features. Models consistently demonstrated that factors such as underlying health conditions and fever greatly influenced predictions. This offers essential guidance for future public health responses, emphasizing the critical need for timely interventions, particularly among vulnerable populations.
While limitations exist — primarily due to the size and scope of our study population — the insights garnered could play a pivotal role in managing and predicting severe cases of COVID-19. Machine learning applied to clinical and genomic data can offer foresight in not just COVID-19, but in upcoming pandemics, enabling healthcare systems to respond rapidly and effectively.
As COVID-19 mutations continue to circulate, our findings suggest the ongoing necessity to integrate genomic understanding into clinical practices, ensuring that healthcare systems are equipped to tackle future challenges posed by not just COVID-19 but other infectious diseases. Understanding these associations further informs vaccination strategies and public health policies aimed at minimizing severe outcomes and, ultimately, saving lives.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)