Unveiling the Secrets of Nanostructures: Breakthroughs in Electrodeposition
2024-11-13
Author: Nur
Introduction
Metallic nanoparticles have become a focal point in modern science, increasingly capturing the attention of researchers and industries alike. Comprising only a few to several thousand atoms, these tiny structures offer unparalleled potential, particularly when used in electrode-coated layers for cutting-edge applications in energy production and catalysis.
Recent Advances in Electrodeposition
Recently, an international consortium spearheaded by scientists from the Institute of Nuclear Physics at the Polish Academy of Sciences has made remarkable strides in understanding how to create these nanostructures through a process known as electrodeposition. Their findings, published in the journal Nano Letters, delve into the intricate dynamics of this method, which is poised to advance fields as diverse as electronics, medicine, and renewable energy technologies.
Understanding Electrodeposition
Electrodeposition is an efficient process that enables the formation of thin nanolayers on electrodes by immersing them in metal salt solutions and applying voltage. The application of electricity prompts ions to migrate and reduce, leading to the growth of a solid layer. However, until now, comprehending the mechanisms at work during this growth has remained elusive.
Investigating Nanolayer Formation
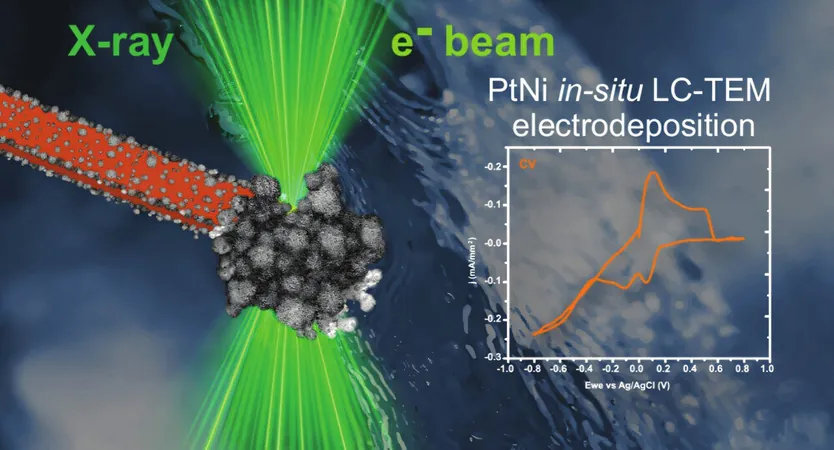
The researchers conducted sophisticated experiments to investigate the formation of a platinum-nickel (PtNi) nanolayer in real-time, harnessing state-of-the-art imaging technologies. By utilizing transmission electron microscopy (TEM), they were able to achieve unprecedented sub-angstrom resolution, allowing them to observe the atomic interactions that occur during nanoparticle formation. Thanks to innovative techniques, they explored the interactions in a liquid medium, overcoming the traditional limitations of TEM, which typically requires dry, ultra-thin samples.
Innovative Experimental Setup
In their experimental setup, the team ingeniously employed a custom imaging chamber fashioned out of two silicon chips with a 50-nanometer-thick SiNx membrane. The electrons passed through this electron-transparent membrane, enabling them to observe not only the growth patterns but also the initial stages of nanoparticle nucleation at the electrode's surface. Surprisingly, they found that nanoparticles could initially form in the surrounding electrolyte before attaching to the electrode—an observation which revealed rich details about electron interactions with water.
Characterization of Nanoparticles
As they explored further, the researchers noted that the formed layers consisted of spherical nanoparticles, approximately tens of nanometers in diameter, with surfaces exhibiting densely branched dendritic structures. This intricate construction is crucial for enhancing the catalytical properties of the nanoparticles, making them extremely valuable for energy applications such as fuel cells.
Collaboration and Further Findings
In collaboration with the prestigious Fritz Haber Institute of the Max Planck Society in Berlin, the team extended their experiments by adjusting the reaction times and voltage rates. They discovered that the alternating cycles of growth and dissolution of nanoparticles lead to stable layers, marking a crucial breakthrough in fine-tuning the electrodeposition process.
Utilizing Advanced Microscopy Techniques
Not stopping there, the researchers also leveraged a unique scanning transmission X-ray microscope (STXM) at Kraków's National Synchrotron Radiation Center SOLARIS to study the oxidation states of the nanoparticles produced. They confirmed that through various techniques, the PtNi layer synthesized was not merely pure metal, but often involved complex oxides, unveiling a deeper understanding of the material's properties at the atomic level.
Conclusion
The challenges of performing such experiments in a liquid environment cannot be understated; however, the team successfully merged high-tech microscopy with innovative chemical processes, yielding information that’s vital for future applications in nanotechnology. As ongoing investigations continue to shine light on the synthesis and growth of nanostructures, this research may lead to the creation of tailored materials for various applications—from more efficient fuel cells to revolutionary medical devices. With the findings pushing boundaries in both practical and theoretical realms, we stand on the brink of a new era in nanotechnology—one where the secrets of the miniature world may very well transform our macro world.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)