Unveiling the Secrets of Cancer Immunotherapy: How Antibodies Fight Back Against Blood Cancers
2025-01-10
Author: Wei
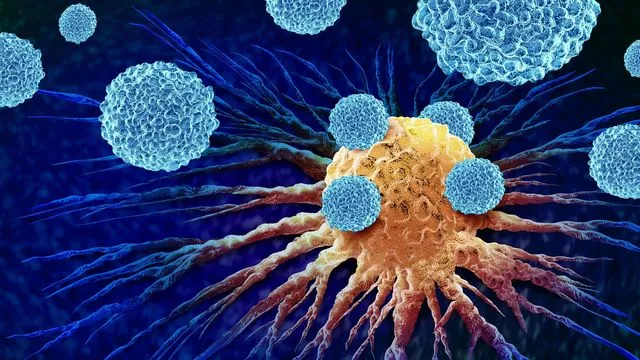
In the battle against blood cancers like chronic lymphocytic leukemia, B cells – critical components of our immune system – can spiral out of control. One promising therapeutic strategy involves using specially designed antibodies to target the CD20 protein that sits atop these rogue B cells. This innovative approach not only marks these cells for destruction but also ignites a cascade of immune responses that ultimately leads to the demise of the cancerous cells.
Immunotherapeutic antibodies have been a cornerstone in the treatment of various tumor diseases for three decades. However, the intricate details of how these antibodies engage with CD20 and initiate subsequent immune reactions remained elusive. As Professor Markus Sauer from the Biocenter at Julius-Maximilians-Universität Würzburg in Bavaria, Germany, mentions, 'While crucial to therapy success, our understanding of this process is still limited.'
A Breakthrough in Understanding Antibody Efficacy
Recent groundbreaking work by a research team led by Professor Sauer heralds a new era in our comprehension of these vital therapies. They have developed a revolutionary super-resolution microscopy technique, allowing scientists to examine the interactions between therapeutic antibodies and target molecules on tumor cells in three-dimensional, molecular detail.
'This advancement enables us to evaluate the effectiveness of the antibodies and aids in designing enhanced therapeutic strategies,' explains Markus Sauer. The newly coined method, LLS-TDI-DNA-PAINT, was recently detailed in the prestigious journal Science. The study, led by Dr. Arindam Ghosh, unveils how this innovative technology operates and highlights early findings that promise to reshape our approach to cancer treatment.
Discovering the ‘Hedgehog’ Effect in B Cells
The Würzburg team conducted an extensive analysis on both fixed and live Raji B cells—an essential cell line derived from a patient with Burkitt's lymphoma and commonly utilized in cancer research. The researchers introduced one of four therapeutic antibodies: RTX, OFA, OBZ, and 2H7, observing significant interactions between these antibodies and the CD20 proteins.
Remarkably, all four antibodies caused CD20 molecules to cluster on the B cell membranes, triggering the complement system and activating immune-mediated cell destruction. Their findings challenge existing paradigms, suggesting that the previously defined categories of therapeutic antibodies—Type I and II—do not hold up under scrutiny.
The research revealed that the antibodies localized selectively at specific membrane areas, notably on tiny membrane extensions known as microvilli. This binding led to a polarization effect, causing the B cells to adopt a unique hedgehog-like shape, with protrusions concentrated on just one side of the cell.
Looking Ahead: New Questions in Immunotherapy Research
What does this mean for future studies? Dr. Ghosh notes, 'Our results indicate that the traditional classifications of therapeutic antibodies are not sustainable.' Historically, it was presumed that Type I antibodies operated via distinct mechanisms compared to Type II. However, the findings from Würzburg's investigations contradict this longstanding belief.
With B cells taking on an appearance reminiscent of hedgehogs, it is hypothesized that these modified cells may facilitate interactions with other immune cells, such as macrophages and natural killer cells. This avenue will be the focus of ongoing research, as scientists seek to validate this hypothesis and uncover new ways of boosting the efficacy of immunotherapies against blood cancers.
In summary, these advances not only augment our understanding of how targeted therapies work but also pave the way for the development of new, more effective treatments that could revolutionize cancer care. As immunotherapy continues to evolve, we may be witnessing the dawn of a new frontier in the fight against cancer. Stay tuned for more updates on this exciting research!



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)