Unveiling the Secrets: How Binding Energy Distributions of Alcohols and Thiols Impact Interstellar Chemistry
2025-09-04
Author: Jia
The Key to the Cosmos: Understanding Binding Energy
Binding energy (BE) stands as a pivotal factor in astrochemical modeling, dictating how various molecules cling to interstellar dust grains and evolve chemically over time. This understanding could redefine our knowledge of molecular formations in the vast expanse of space.
Going Beyond Conventional Models
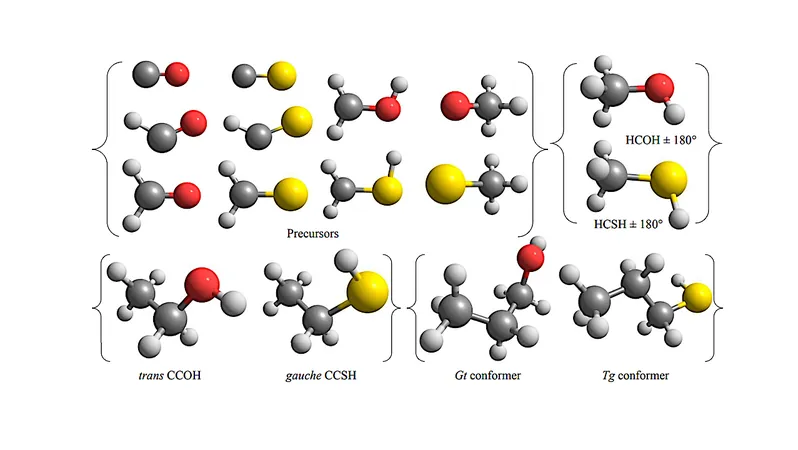
Traditional models have tended to simplify things by using fixed values for BEs, which often disregards the rich diversity seen in the adsorption sites available. In groundbreaking research, scientists have shifted gears by examining the BEs of monohydric alcohols, thiols, and their potential precursors, such as aldehydes and thioaldehydes.
A Nuanced Approach: Distribution of Binding Energies
By implementing a range of BEs instead of a single figure, this study encapsulates the variation in how these molecules interact with surfaces. Quantum chemical calculations reveal a spectrum of BE values, enhancing our understanding of molecular diffusion and the complex surface chemistry involved.
Insights into Cosmic Reactions
The research outlines a significant trend: oxygen-containing molecules tend to display higher binding energies than their sulfur counterparts, an insight that may profoundly influence how these species react and bond in space. This understanding is crucial for piecing together the pathways leading to the synthesis of larger, more complex molecules.
Transforming Astrochemical Models
By integrating these binding energy distributions into astrochemical models, researchers have discovered notable changes in predicted elemental abundances, ushering in a new paradigm for future exploration and study in astrochemistry.
Visualizing Cosmic Structures
Among the findings is the identification of the most stable amorphous solid water (ASW) clusters, with detailed illustrations available for further examination. Each atom is represented clearly: red for oxygen, white for hydrogen, grey for carbon, and yellow for sulfur.
A Collaborative Endeavor
This pioneering research is a collective effort by scientists including Arghyadeb Roy, Ankan Das, Milan Sil, and others, detailing their findings in a comprehensive 14-page study set to be published in the prestigious Life Sciences in Space Research journal.
The Future of Astrochemistry Awaiting Discovery
As we continue to decode the intricate dance of molecules in the cosmos, understanding binding energy distributions offers a gateway to unraveling the mysteries of star formation and the potential for life beyond our planet.
 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)