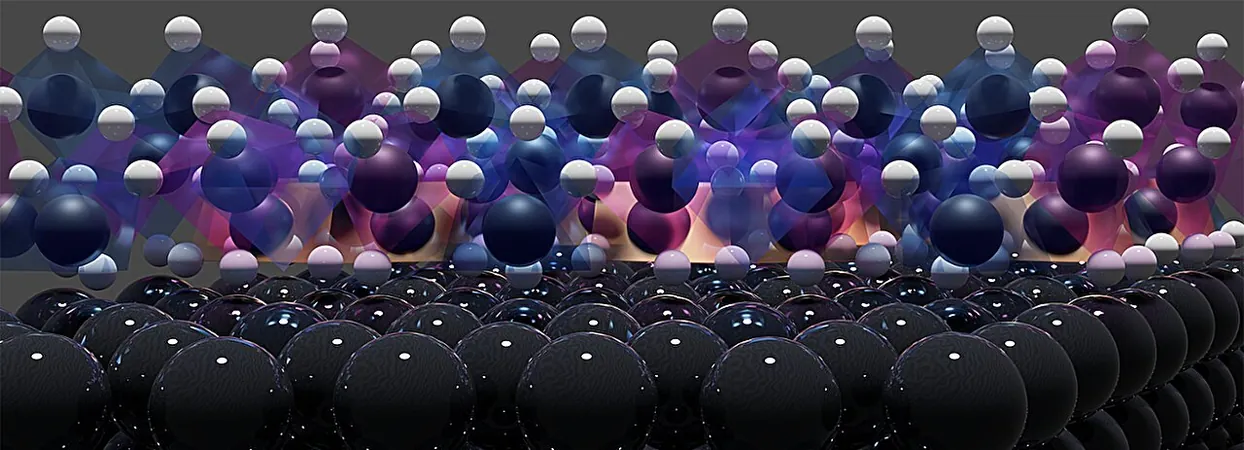
Unveiling the Mystery: The Shocking Truth Behind Platinum Electrode Corrosion
2025-01-24
Author: Mei
Scientists from Leiden University and the Department of Energy's SLAC National Laboratory have made a groundbreaking discovery that may revolutionize green technology: they’ve unveiled the mysterious cause of the rapid corrosion plaguing platinum electrodes. This revelation not only sheds light on a problem that has mystified researchers for decades but also paves the way for more cost-effective green hydrogen production and reliable electrochemical sensors.
Published in the esteemed journal *Nature Materials*, the study reveals a paradox in electrochemistry. Typically, negatively polarizing metals serves as a protective measure against corrosion; however, platinum electrodes showcase a disturbing anomaly — they can deteriorate swiftly under similar conditions. This poses a significant challenge, as electrolyzers and numerous electrochemical devices heavily depend on these negatively charged platinum electrodes plunged in electrolyte solutions—often resembling saltwater.
Dimosthenis Sokaras, principal investigator at SLAC, clarifies, “Just because platinum is known for its stability doesn’t mean it is immune to degradation.”
What’s Behind the Degradation?
The research initially proposed two primary theories to explain the corrosion phenomenon. One hypothesis suggested that sodium ions within the electrolyte penetrate the platinum’s atomic structure, forming platinides—structures where platinum atoms meld with positively charged sodium ions, resulting in degradation. Meanwhile, another theory implicated both sodium and hydrogen ions, positing a collaborative effort in generating platinum hydrides.
A First-Hand Look at Corrosion
To investigate the corrosion process, the research team aimed to observe platinum during its operational phase, while simultaneously producing hydrogen gas. They utilized SLAC's advanced Stanford Synchrotron Radiation Lightsource, where high-energy-resolution X-ray spectroscopy techniques were developed specifically to penetrate the electrolyte, allowing scientists to focus on subtle changes occurring in the platinum electrode in real-time.
SLAC scientist Thom Hersbach remarks, “High-energy-resolution X-ray absorption spectroscopy was our best tool under these challenging experimental conditions.”
Additionally, the researchers innovated a specialized pump and growth cell that efficiently cleared hydrogen bubbles forming on the electrode surface—bubbles that could interfere with the X-ray observations.
Unmasking the Culprit: Platinum Hydrides
Bringing all these sophisticated techniques together enabled the team to capture unprecedented images of platinum undergoing active corrosion. Their analysis revealed that platinum hydrides were indeed the primary offenders behind the degradation. Prior to the experiment, the researchers suspected the existence of hydrides, but it required extensive data analysis over several years to substantiate their hypothesis. “We had to rigorously assess and iterate our experiments to gain a deeper understanding of the processes at play,” Hersbach added.
By employing computational models to simulate the expected X-ray spectra of platinum hydrides and platinides, they ultimately confirmed that the experimental results could only be attributed to platinum hydrides.
Sokaras emphasized the achievement, stating, “With the groundbreaking advancements in X-ray techniques at SSRL, coupled with modern computational power, we are now positioned to tackle scientific enigmas that have remained unsolved for decades.”
The implications of these findings are substantial. With the understanding of what triggers platinum corrosion, researchers can now devise strategies to mitigate this persistent issue in electrolyzers and various other electrochemical devices, thereby enhancing their reliability and longevity. Koper concluded, “This project truly exemplifies the necessity of collaborative expertise in scientific endeavors.”
Could this discovery be the key to unlocking a new era of sustainable energy? Stay tuned — the future of green hydrogen production might just be on the horizon!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)