Unveiling the Mysteries of Protein Behavior: How Proteins Restructure Themselves to Create Essential Substances
2024-09-23
Introduction
Recent groundbreaking research has revealed astonishing insights into how proteins, specifically the protein 'MIPS', can self-restructure to adapt for crucial biological functions. When MIPS becomes activated, its chaotic internal structure transitions into a well-defined formation equipped with specialized roles. This transformation is vital in the production of inositol, commonly referred to as vitamin B8, which is indispensable for several critical bodily processes.
Research Collaboration
A collaborative team from Martin Luther University Halle-Wittenberg (MLU) and the National Hellenic Research Center in Greece has successfully tracked this re-structuring process for the first time. Their findings, published in the prestigious Proceedings of the National Academy of Sciences, suggest that this phenomenon might be widespread among similar proteins.
Significance of Protein Behavior
Understanding protein behavior is crucial because proteins govern all essential life processes, including growth and metabolism. A fundamental principle in protein biology states that a protein's function is dictated by its structure. Even minor structural impairments can render a protein nonfunctional, potentially leading to severe health issues such as metabolic disorders or neurodegenerative diseases.
Dynamic Proteins
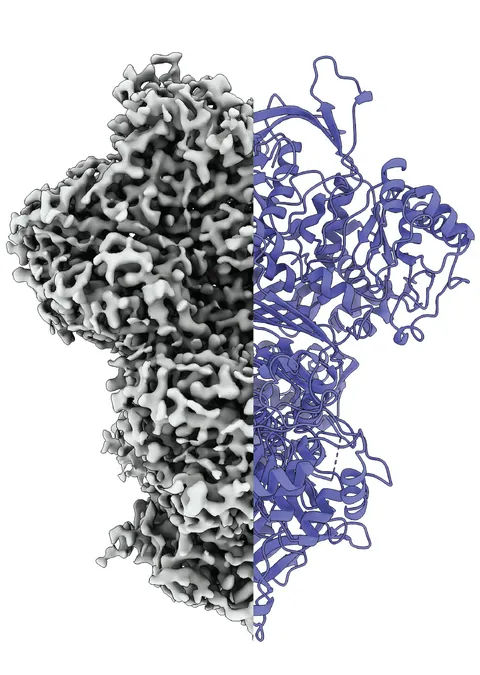
Interestingly, many proteins lack a stable structure, operating instead in a dynamic and adaptable manner. These proteins, often difficult to study, undergo conformational changes based on their surrounding environment. As Professor Panagiotis Kastritis, a biochemist at MLU, highlights, traditional methods that isolate proteins cannot capture their natural behaviors. His innovative approach allows proteins to be analyzed under conditions that closely mimic their natural state.
Methodology
In their groundbreaking study, the researchers focused on the fungus Thermochaetoides thermophila, using it as a model organism. MIPS was at the center of their research. This protein is integral to producing inositol, a vital compound the body synthesizes but does not classify as a true vitamin due to its endogenous production.
Observations and Findings
Toni Träger, a co-researcher who previously examined MIPS for his master's thesis, elaborates, “MIPS is part of an extended metabolic network leading to inositol production.” Utilising advanced cryo-electron microscopy, the team observed MIPS in action, identifying at least three states of the protein: a disordered form, an ordered form, and a unique intermediate state whose purpose remains unclear. Professor Kastritis speculates that this intermediate state might facilitate water absorption or perform some other critical function during metabolic processes.
Broader Implications
To deepen their inquiry, the researchers also investigated whether proteins analogous to MIPS displayed similar conformational characteristics. This analysis encompassed over 340 additional isomerases, which are part of the same protein family. They discovered compelling evidence indicating that these proteins likely exhibit related behavior patterns.
Conclusion
The implications of these findings extend beyond academic curiosity. A more profound understanding of these metabolic pathways and the proteins intertwined within them could revolutionize therapeutic strategies, paving the way for innovative treatment options for various diseases. As Professor Kastritis aptly concludes, “Our work is a significant first step towards unlocking new therapeutic avenues.” This research not only sheds light on the intricate dance of proteins but also holds promise for future breakthroughs in medical science. Stay tuned for more exciting discoveries from the world of biochemistry!




 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)