Unveiling the Hidden Power of Mitochondria: How Stress Reshapes Cellular Identity
2025-08-04
Author: Wei
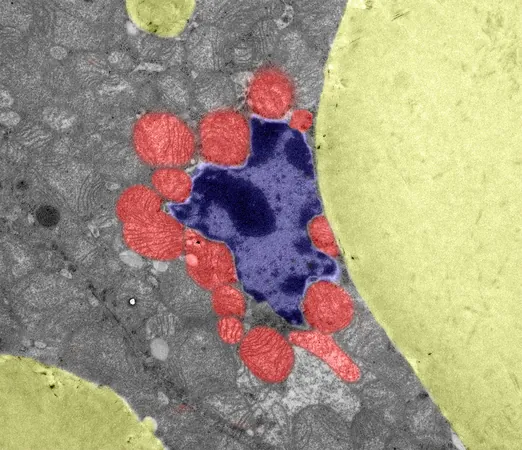
Mitochondria, the powerhouse of our cells, do more than just generate energy—they are crucial players in how cells cope with stress. When mitochondrial function declines, especially in energy-intensive areas like brown fat, the whole organism must adapt in unexpected ways.
In an intriguing new study led by Professor Dr. Aleksandra Trifunovic from the CECAD Cluster of Excellence for Aging Research, researchers explored this phenomenon using a mouse model with flawed mitochondrial quality control. Rather than simply shutting down under stress, cells in brown fat orchestrate a remarkable metabolic response, repurposing key enzymes to produce a compound known as D-2HG.
Published in Nature Metabolism, this groundbreaking research reveals D-2HG's dual role. Previously linked to cancer progression, in this context it acts as a crucial adaptation mechanism by modulating how DNA is organized within the nucleus, influencing gene expression, and even reshaping the nuclear envelope.
"What’s fascinating is that D-2HG, often seen as detrimental, may actually foster cellular adaptability," says lead author Dr. Harshita Kaul. "We’re just beginning to unravel the complex signaling pathways between mitochondria and the rest of the cell during stress."
The study also sheds light on the less recognized functions of mitochondria, specifically their role in maintaining brown fat’s healthy function. This unique fat type regulates body temperature and metabolism by converting energy into heat. When mitochondria fail, brown fat starts to lose its special characteristics, beginning to look more like regular white fat—a process romantically dubbed "whitening."
Elevated levels of D-2HG were found to accelerate this whitening effect, marking a significant shift in cellular identity. "The rewiring driven by D-2HG seems to run parallel to a broader stress response we term the mitochondrial integrated stress response," explains Professor Trifunovic.
What stands out is how D-2HG fosters a connection between mitochondrial dysfunction and nuclear mechanics—a remarkable form of cross-communication that challenges our traditional understanding of cellular adaptation. This research opens the door to new diagnostic avenues, potentially revolutionizing our approach to metabolic disorders and aging-related diseases.
Currently, the researchers are investigating whether this pathway is active in other vital tissues, such as the heart and brain, and how it might be harnessed for therapeutic purposes.



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)