Unveiling the Crucial Role of RNA Modifications in Cancer: Can We Target Them for Therapy?
2024-10-25
Author: Sarah
Introduction
Cancer remains one of the most pressing health challenges worldwide, deeply entwined with misregulated gene expression driven by both genetic mutations and epigenetic alterations, which include various RNA modifications. A recent comprehensive review in Frontiers of Medicine has shed light on the significant roles that these RNA modifications play in the onset and progression of cancer, particularly highlighting N6-methyladenosine (m6A) as the most prevalent internal modification in messenger RNA (mRNA).
The Role of m6A Modification
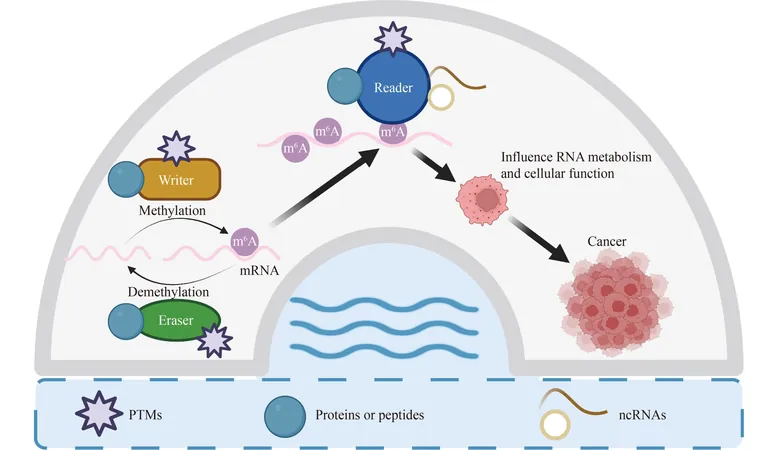
The m6A modification is introduced by specific enzymes known as 'writers,' predominantly methyltransferases such as METTL3, METTL14, and WTAP. Conversely, 'erasers' like FTO and ALKBH5 remove these modifications. A class of proteins known as 'readers,' including YTHDF proteins, recognize m6A sites and initiate downstream effects that are vital for several biological processes. The review pointedly notes that the levels of m6A and its regulatory proteins often show significant alterations in cancerous cells, contributing to critical cancer traits such as uncontrolled proliferation, enhanced survival, metastasis, and even resistance to therapies.
Regulatory Subunits and Post-Translational Modifications
Furthermore, regulatory subunits and post-translational modifications (PTMs)—such as acetylation, SUMOylation, and phosphorylation—further refine the function of m6A regulators. For example, the acetylation of ALKBH5 by KAT8 has been shown to amplify its demethylating effects, thus fostering tumorigenesis. Moreover, the activity of METTL3 can be modified through SUMOylation, impacting cancer cell growth and invasion.
Therapeutic Potential of Targeting m6A Regulators
Importantly, the review explores the therapeutic potential of targeting m6A regulators in cancer treatment. Experimental small molecule inhibitors aimed at the writers, erasers, and readers of m6A have exhibited encouraging results in preclinical settings. These inhibitors can significantly alter RNA metabolism, affecting translation and decay processes, which may subsequently hinder malignant cancer cell behaviors.
Other RNA Modifications in Cancer
In addition to m6A, the review touches on other RNA modifications that are gaining recognition for their roles in gene regulation. Modifications such as N1-methyladenosine (m1A), 5-methylcytidine (m5C), N7-methylguanosine (m7G), N6,2'-O-dimethyladenosine (m6Am), and 5-formylcytidine (ac4C) also play crucial roles in cancer biology. For instance, m1A is crucial for mRNA stability and translation with implications tied to writers like TRMT6 and erasers like ALKBH3, while m5C affects RNA stability, influenced by writers like NSUN2 and erasers TET1.
Conclusion
The review concludes by emphasizing the necessity of comprehensively understanding the intricate relationships among various RNA modifications and their regulators in the cancer landscape. A systems biology approach might uncover how these modifications interact, compete, or collaborate to drive cancer phenotypes. Despite advancements in m6A research, other RNA modifications and their roles in cancer are still in the exploratory stages, leaving ample space for further investigation into their mechanistic pathways and therapeutic potential.
Summary
In summary, this detailed exploration of RNA modifications in cancer underlines a critical area for future research and therapeutic development. As scientists delve deeper into this burgeoning field, the hope is that targeting RNA modifications could pave the way for innovative cancer treatments, potentially changing the landscape of how we approach cancer therapy. Stay tuned—this could be a game-changer in the fight against cancer!
 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)