Unveiling the Chemical Mysteries of Superheavy Elements Moscovium and Nihonium: A New Era of Chemistry Begins!
2024-11-05
Author: Nur
Introduction
In a groundbreaking scientific achievement, an international team of researchers led by the GSI Helmholtzzentrum für Schwerionenforschung in Darmstadt, along with partners from Johannes Gutenberg University Mainz and the Helmholtz Institute Mainz, has successfully characterized the chemical properties of two artificially produced superheavy elements: moscovium (element 115) and nihonium (element 113).
Chemical Analysis of Superheavy Elements
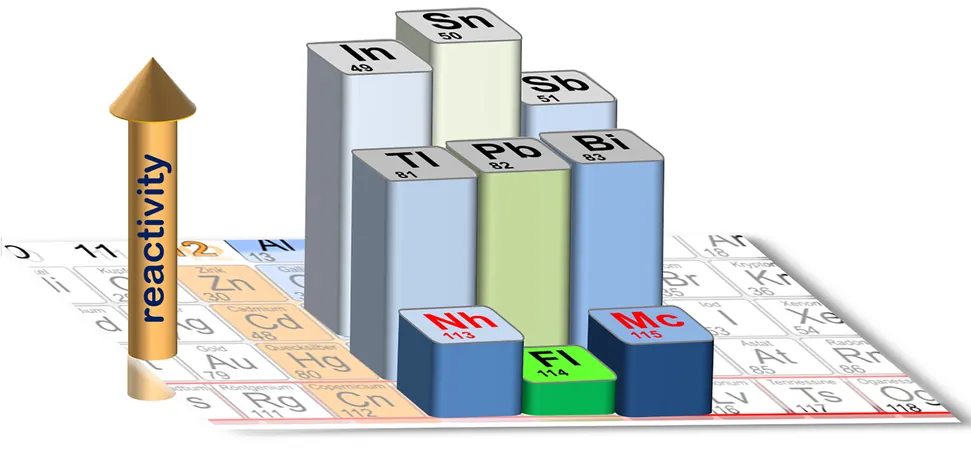
This momentous study marks moscovium as the heaviest element ever subjected to chemical analysis. Through meticulous experimentation, the researchers discovered that these newly characterized elements exhibit a remarkable level of reactivity, surpassing the previously studied element, flerovium (element 114). These findings are detailed in the latest issue of the journal *Frontiers in Chemistry*, shedding light on the complex behavior of elements in the extreme regions of the periodic table.
Relativistic Effects in Superheavy Elements
The pursuit of understanding superheavy elements is driven by the unique implications of their physical and chemical properties. As elements get heavier, the immense number of protons in their nuclei leads to an increase in electron velocity, a phenomenon that necessitates the application of Einstein's theories of relativity. This acceleration imparts additional mass to electrons, affecting their interactions with other matter.
Impact on Technology
For instance, lead (element 82) exhibits these relativistic effects, playing a crucial role in technologies like lead-acid batteries. In contrast, its neighboring elements—thallium and bismuth—display markedly different behaviors. Flerovium, discovered and investigated over the past two decades, shows surprising properties, transforming easily into gas and exhibiting lower chemical reactivity compared to lead.
Research Findings on Nihonium and Moscovium
To deepen this understanding, researchers turned their attention to nihonium and moscovium. While initial hints regarding nihonium's chemistry were previously reported, the investigation of moscovium had yet to be accomplished, mainly due to its fleeting existence—one of its isotopes lasts a mere 0.2 seconds.
Experimental Methodology
The collaborative team's efforts at GSI Helmholtzzentrum have led to significant breakthroughs. They found that both nihonium and moscovium are even more chemically active than flerovium, highlighting the influence of nuclear charge on chemical behavior. Their experiments involved producing moscovium-288 by bombarding americium-243 with calcium-48 ions, a process that requires high-energy environments and innovative detection techniques.
Chemical Separation and Detection Techniques
The researchers developed a sophisticated setup for chemical separation and detection, leveraging gas chromatography methods that are vital for pinpointing the elusive properties of these superheavy elements. Remarkably, they successfully registered four moscovium atoms and 14 nihonium atoms, providing valuable insights into their bonding behaviors with quartz and gold, which differ from their lighter homologs.
Inertness and Relativistic Effects
One of the most fascinating discoveries relates to the inertness associated with relativistic effects, showing that superheavy elements exhibit less reactivity compared to their lighter counterparts—phenomena linked to closed electron shells typical of noble gases.
Future Implications and Research
This study not only sets a record for the heaviest chemically analyzed element but also engenders new discussions about the applications of superheavy elements. As we continue to innovate technologically, the potential for these newly characterized materials in batteries, medical devices, and beyond remains a tantalizing possibility for the future.
Conclusion
With the FAIR (Facility for Antiproton and Ion Research) under construction in Darmstadt, future researchers are poised to explore even more about these elements, potentially revolutionizing how we understand chemistry and material science in the years to come. As we push the boundaries of what is possible, we may soon find ourselves harnessing the unique properties of moscovium, nihonium, and their ilk in ways we can barely imagine today!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)