Unraveling the Deadly Secrets of the Ebola Virus: A Breakthrough in Viral Research
2025-03-26
Author: John Tan
In a chilling reminder of past health crises, the Ebola virus surged in West Africa six years before the world was gripped by the COVID-19 pandemic. This marked the first encounter for many with Ebola, a virus identified back in 1976 that has since caused over 20 significant outbreaks. Fortunately, none have compared to the global scale of coronavirus—yet the threat of Ebola remains ever-present.
Ebola is notorious for causing severe hemorrhagic fever, with a staggering fatality rate that hovers around 50%. Despite its historical severity, the virus has not been eradicated, and researchers warn that without further investigation and development of effective treatments, it could lead to another outbreak that could shake the world.
A major barrier in combating the virus has been its elusive structure, particularly the regulatory mechanisms of its nucleocapsid, the protein shell crucial for the virus's genome replication and transcription. Understanding this structure is vital for developing antiviral strategies.
Driven by this necessity, an innovative team from Kyoto University conducted groundbreaking research, capturing the first-ever high-resolution images of the Ebola virus nucleocapsid. They employed single-particle cryo-electron microscopy, a cutting-edge technique that allows scientists to visualize molecular structures with astonishing detail by freezing samples almost instantaneously.
Their exciting results were published in the reputable journal Nature Communications. Corresponding author Takeshi Noda proclaimed, "Using cryo-electron microscopy, we were able to view the nucleocapsid structure at an unprecedented resolution of 4.6 angstroms—the first time this level of detail has ever been achieved."
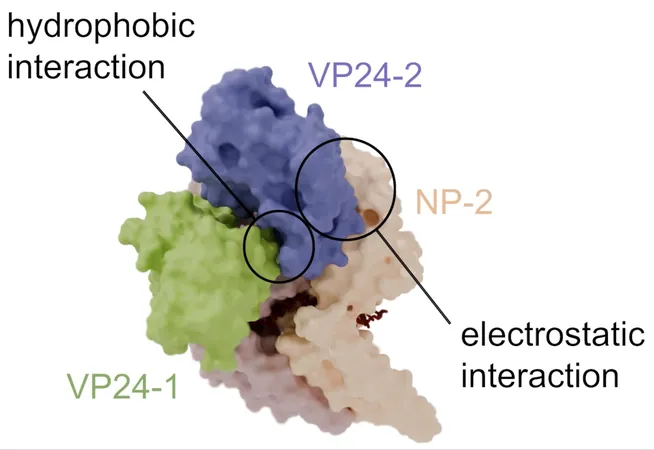
With this newfound clarity, the researchers unveiled the intricate interactions within the nucleocapsid. They examined two vital proteins: NP (nucleoprotein) and VP24 (viral protein), both pivotal for assembling the nucleocapsid and regulating RNA synthesis and intracellular transport. Their study revealed that the spiral structure of the NP-RNA complex showcased a remarkable binding pattern—two VP24 molecules were found interacting with two NP molecules in varied formations.
This sophisticated interplay is crucial. VP24 serves a dual purpose: one molecule inhibits viral RNA production while another facilitates the assembly and transport of the nucleocapsid. This dynamic capability allows the virus to switch between crucial processes of genome replication and the packaging of new virions.
"Our high-resolution structure of the Ebola virus nucleocapsid sheds light on intricate molecular relationships and how these interactions govern vital functions," Noda explained. "This knowledge is essential for devising targeted antiviral therapies aimed at disrupting nucleocapsid assembly and its functions."
As scientists continue to pore over these findings, the significance extends beyond mere academic interest. These insights could enhance preparedness and response strategies for future viral outbreaks, not just for Ebola but for other emerging infectious diseases that may pose similar threats.
This revolutionary study marks a crucial milestone in the ongoing battle against one of nature's most devastating pathogens. With continued research and innovation, the world may one day turn the tide against Ebola and similar viruses, safeguarding public health for generations to come.



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)