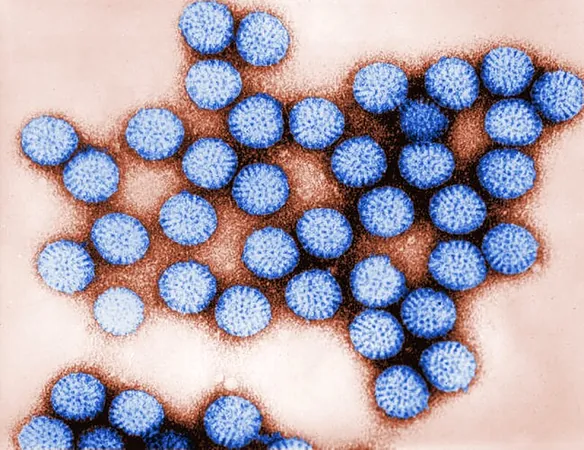
Unraveling Rotavirus: How NSP4 Protein Disrupts Calcium Signaling and Influences Severity of Infection
2025-01-18
Author: Mei
In a groundbreaking study published in *Science Advances*, researchers from Baylor College of Medicine, in collaboration with other esteemed institutions, have shed light on the mechanisms by which rotavirus, a leading cause of acute gastroenteritis in children, inflicts illness. This research is pioneering in revealing the critical role of the rotavirus protein NSP4, which not only disrupts calcium signaling within infected cells but also affects adjacent uninfected cells, a phenomenon that significantly impacts the severity of rotavirus disease.
Dr. Joseph Hyser, the study's corresponding author and an associate professor of molecular virology and microbiology at Baylor, emphasized the global health threat posed by rotavirus: "This virus is responsible for approximately a quarter of severe pediatric gastroenteritis cases, leading to nearly 500,000 child deaths annually." Although measures such as oral rehydration therapy and live-attenuated vaccines have diminished the prevalence of rotavirus infections, there is ample scope for further advancements.
The team delved deeper into NSP4's functionalities, hoping to pinpoint novel therapeutic strategies. Previous research indicated that rotavirus could induce atypical calcium signals—termed "intercellular calcium waves"—that emanate from infected to neighboring uninfected cells, increasing the virulence of the virus. However, the specifics surrounding how rotavirus initiated these signals remained unclear until now.
Utilizing various experimental approaches—including lab-grown cells, intestinal organoid cultures, and animal models—the researchers focused on assessing the role of NSP4 in triggering calcium waves connected to disease severity. They discovered that the production of calcium waves could solely be attributed to NSP4’s presence—the expression of this protein was sufficient to elicit waves even without the rotavirus itself.
Strikingly, NSP4 derived from attenuated (weakened) strains of rotavirus produced significantly fewer calcium waves compared to those from virulent strains. When NSP4 from the attenuated strains was inserted into virulent strains, it curtailed the generated calcium waves and lessened the ensuing diarrheal symptoms in animal models.
Dr. Hyser elaborated, "We found a direct correlation between rotavirus's ability to generate calcium waves and NSP4. The presence of NSP4 alone could trigger intercellular calcium signaling; moreover, the severity of rotavirus disease appeared linked to this capability."
Moreover, these calcium waves not only regulate viral replication but also initiate immune responses, suggesting that calcium dysregulation might be a mechanism for the host cells to recognize viral infections. This insight opens avenues to potentially target NSP4 across different types of viruses that may exploit similar calcium-disrupting proteins.
The implications of this research extend beyond rotavirus, as targeting mechanisms similar to NSP4 could provide opportunities to combat various viral infections that manipulate calcium signaling pathways. As the global health community continues to grapple with infectious diseases, this study highlights a potential breakthrough in our fight against one of the most pervasive childhood illnesses.
Other contributors to this impactful work include J. Thomas Gebert, Francesca J. Scribano, and several colleagues affiliated with institutions such as Indiana University and Stanford University School of Medicine.
Stay tuned as scientists explore how these findings could revolutionize prevention and treatment strategies for rotavirus infections and possibly other viral diseases!

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)