Unmasking the Cancer Culprit: PAD2 Enzyme's Dastardly Role in Pancreatic Cancer Progression
2025-08-27
Author: Wei
A Breakthrough Discovery in Pancreatic Cancer Research
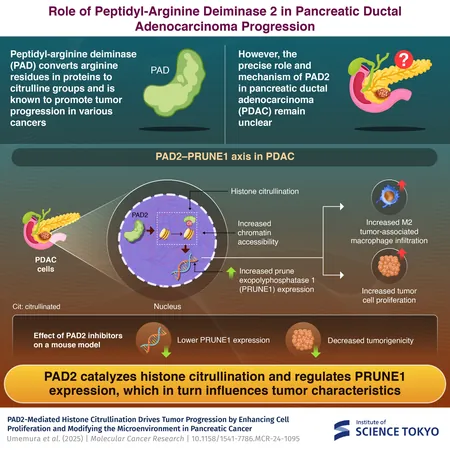
In a groundbreaking study from the Institute of Science Tokyo, researchers have unveiled the role of the peptidyl-arginine deiminase 2 (PAD2) enzyme as a driving force behind pancreatic ductal adenocarcinoma (PDAC). This enzyme transforms arginine residues in histone proteins to citrulline, effectively fueling the proliferation of cancer cells.
The Challenge of Pancreatic Cancer
Pancreatic cancer remains one of the most difficult cancers to treat. With an alarming tendency for metastasis and resistance to therapies, PDAC is notorious for leading to poor patient outcomes. Researchers have been racing against time to find new treatments by delving into the genetic makeup and protein expressions of these aggressive tumors.
The Role of Histone Citrullination
Recent studies have brought to light the role of PAD enzymes, which are known to regulate gene expression across various cancers. By converting arginine to citrulline, PADs enhance access to DNA strands, thus promoting tumor growth. However, the exact functioning of PAD2 in PDAC was unclear—until now.
Unlocking the Mechanism of PAD2
Under the guidance of Professor Shinji Tanaka, the research team utilized cutting-edge genetic manipulation techniques to expose the intricacies of PAD2's function in PDAC. Their findings, published in the journal Molecular Cancer Research on June 26, 2025, bring us one step closer to targeted therapies.
Crucial Findings from Patient Tissue Samples
The researchers initially conducted immunohistochemistry staining on tissue samples from PDAC patients to assess the prevalence of histone citrullination. They discovered that this phenomenon was significantly greater in PDAC tissues compared to normal pancreatic tissues and correlated with a lower survival rate.
The Impact of PAD2 Manipulation
The team then created cell lines with either increased or decreased levels of PAD2. Experimentation revealed that cancer cells lacking PAD2 exhibited slower proliferation, while those with overexpressed PAD2 grew at an accelerated rate.
Identifying Gene Targets
To delve deeper, the researchers performed RNA-sequencing on PAD2-knockdown cells, revealing eight significantly downregulated genes. Among them were PRUNE1 and E2F transcription factor 1, both of which were upregulated in cells overexpressing PAD2, indicating that this enzyme directly influences their expression.
In Vivo Confirmation of Tumor Growth
In an impressive series of experiments, they injected mice with PAD2-overexpressing or PAD2-knockdown cells to observe tumor formation. Results showed a marked increase in tumor growth in mice with PAD2-OE cells, along with higher levels of histone citrullination.
A Promising Pathway for Treatment
Tanaka noted, "When treated with PAD inhibitors like Cl-amidine and AFM-30a, a PAD2-specific inhibitor, lower PRUNE1 levels were observed, and tumor growth in mice was significantly hindered.” This study lays the groundwork for innovative therapies targeting PAD2, potentially revolutionizing treatment for this deadly cancer.
Conclusion: A Future of Hope
The findings underscore the pivotal role of PAD2 in PDAC development and highlight how targeting its activity may pave the way for groundbreaking epigenetic therapies against one of the most daunting cancers we face.

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)