Unlocking Viral Secrets: How Poxvirus Manipulates Ribosome Function to Multiply
2025-06-02
Author: Nur
In a groundbreaking study published in *Nature Microbiology*, researchers from Northwestern Medicine have revealed stunning insights into how the poxvirus exploits its host's cellular machinery to replicate and spread.
Poxviruses, notorious for causing lethal diseases like smallpox, rely heavily on the host's ribosomes—tiny protein factories within cells—to transform their viral mRNA into proteins. Despite the crucial role ribosomes play, the exact mechanisms through which poxviruses commandeer this protein synthesis system have remained enigmatic, according to Dr. Derek Walsh, the study's senior author and a professor of Microbiology-Immunology.
"It's fascinating that all viruses, even the remarkable DNA poxviruses which have unique self-sufficient traits, must access host ribosomes for their survival," Walsh stated.
The team employed RNA sequencing and polysome profiling to analyze infected cells, unveiling a complex interaction between viral and host mRNAs during the infection process. According to Walsh, as the infection progresses, the virus triggers a phenomenon known as 'host shutoff' that prioritizes the translation of viral mRNA. Notably, some host mRNAs continue to be translated, though through different, unconventional initiation methods distinct from those of viral mRNAs.
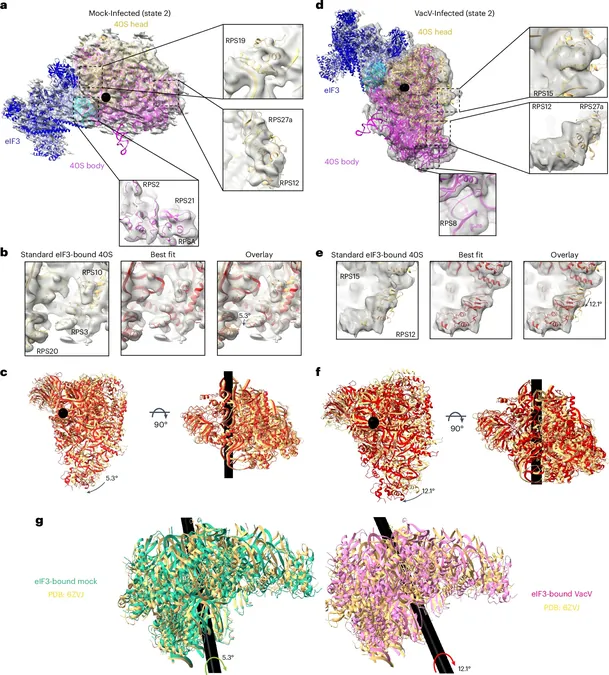
By utilizing cutting-edge cryo-electron microscopy in collaboration with the University of Utah, the researchers observed significant structural modifications in cells infected by the poxvirus. Walsh explained, "Poxvirus infection alters the movement of the 40S head domain during the initiation phase of translation."
Additionally, it was discovered that the virus cleverly employs specific cellular components like the ribosomal protein RACK1 and eukaryotic Initiation Factor eIF3 to enhance its protein synthesis.
These findings shed light on the intricate ways viruses can manipulate cellular processes, highlighting the potential for developing innovative antiviral therapies aimed at disrupting these viral strategies. Walsh remarked, "This research reveals the nuances of how poxviruses can modify ribosomal function and underscores the complexity of both host and viral translation during late-stage infections."
Looking ahead, Walsh and his team are set to explore the role of RACK1 further, along with investigating 79 other ribosomal proteins to deepen their understanding of viral replication.
As they continue this vital research, the implications for combatting poxvirus and similar viral threats could be groundbreaking, possibly steering the future of antiviral treatments.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)