Unlocking the Secrets of Telomeres: How New Discoveries Could Combat Aging and Cancer
2025-04-17
Author: Li
Revolutionary Study Reveals Telomere Regulation Mechanisms
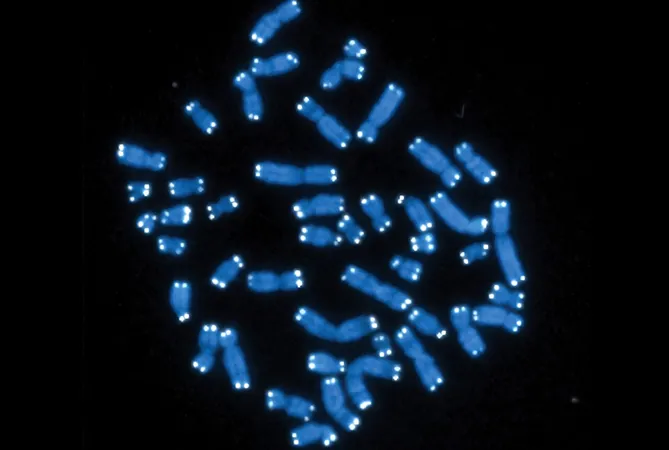
A groundbreaking study from Weill Cornell Medicine sheds light on a vital process that ensures the health of our cells by maintaining the crucial end caps of chromosomes, known as telomeres. Published in *Nucleic Acids Research* on April 17, this research uses yeast to explore how specific protein interactions regulate the enzyme telomerase, thus preventing uncontrolled cell division and premature aging.
The Role of Telomeres in Cell Division
Before a cell divides, it replicates its double-stranded DNA. However, the replication process struggles when it reaches the telomeres. These protective caps safeguard genetic material and typically shorten with age. Here, telomerase steps in, creating an overhang where one DNA strand is slightly longer than the other. A process called fill-in synthesis then aims to complete the shorter strand, ensuring stability. Without proper execution, however, the cell's repair enzymes may misinterpret the structure as damaged, potentially leading to cell death.
Key Players Identified in Telomere Maintenance
The research zeroes in on the three-protein CST complex and the DNA polymerase α/primase (PP) complex, both critical for telomere upkeep. Senior author Dr. Neal Lue emphasizes, "DNA polymerase α is recruited to chromosome ends, forming an assembly with the CST complex, which regulates telomerase and shields chromosome ends from harmful repairs."
Yeast Insights Into Human Biology
Using yeast, a model organism that simplifies the study of fundamental biological processes, the researchers could isolate how these proteins function. Earlier studies by Dr. Lue's team had shown that CST stimulates polymerase α activity, but the intricacies of their interactions were initially unclear.
Mutations Reveal Critical Interactions
When a structure indicating how human CST interacts with PP was reported, Dr. Lue's team collaborated with the Spanish National Cancer Research Centre to draw parallels with yeast protein complexes. They introduced mutations that disrupted CST-PP interactions, leading to fascinating discoveries: in some cases, telomeres grew longer without damage, while in others, growth rates slowed dramatically, leading to varied telomere lengths and excess single-stranded DNA overhangs.
Implications for Aging and Cancer Therapies
These findings have profound implications for understanding telomere biology disorders, such as Coats plus syndrome, where patients experience premature aging and abnormalities in eye and bone health. Dr. Lue notes that mutations increasing telomerase activity are prevalent in various cancers, necessary for cancer cells to bypass aging markers by extending short telomeres.
A Target for Future Cancer Treatments
The potential to manipulate CST protein activity could pave the way for novel cancer therapies. By targeting these proteins with specific drugs, researchers may inhibit the growth of cancer cells by adjusting telomere lengths and their protective status. Such advancements could also aid patients struggling with resistance to existing cancer treatments, offering hope in the battle against these diseases.



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)