Unlocking the Secrets of Reproductive Hormone GnRH: New Insights into its Receptor Activation
2025-06-24
Author: Yu
Back in 1977, Roger Guillemin and Andrew Schally were celebrated with a Nobel Prize for their groundbreaking work on gonadotropin-releasing hormone (GnRH), a pivotal player in our reproductive health. Fast forward to today, and the GnRH receptor (GnRHR) is still a hot topic in the world of biomedical research.
GnRHR is crucial for treating reproductive issues like infertility and combating prostate cancer. The market leader among therapies? Leuprolide, a peptide version of GnRH, expertly targeting this vital receptor. However, its elusive structure has left scientists puzzled—until now.
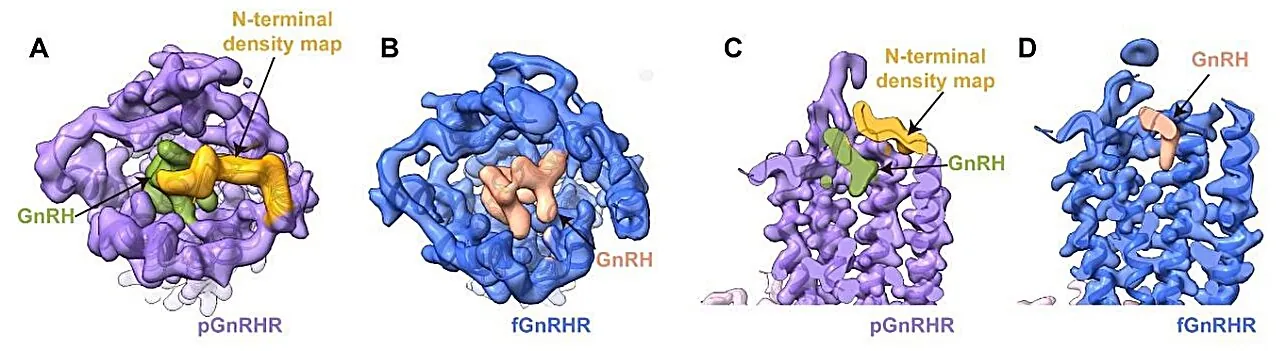
In an exciting new study published in the "Proceedings of the National Academy of Sciences," a research team led by Duan Jia and Xu Huaqiang from the Shanghai Institute of Materia Medica has unveiled high-resolution cryo-electron microscopy (cryo-EM) structures of GnRHR from two species: the African clawed frog and pigs, whose receptors are remarkably similar to ours. Their findings shed light on how GnRH activates its receptor, an intricate dance that was largely shrouded in mystery.
The cryo-EM structures were revealed at impressive resolutions of 2.67 Å and 3.18 Å for the frog and pig complexes, respectively. The researchers found that GnRH adopts a distinctive "U-shaped" formation, intricately inserting itself deep into the receptor's orthosteric pocket, with both ends interacting with crucial areas in the receptor.
Through a detailed comparative analysis, the study highlighted conserved residues critical for binding, including K3.32, Y6.51, and Y6.52, which stabilize the hormone through hydrogen bonds and interactions vital for function.
Moreover, when mutations occurred in these key areas, GnRH signaling was significantly impaired, underscoring their importance. As GnRH binds, it triggers significant structural shifts in the receptor, including an outward flip of the N-terminus and a repositioning of Transmembrane segment 6, which is essential for Gq protein binding.
The researchers meticulously compared active versus inactive receptor states, identifying crucial molecular switches that lead to activation. They also explored structure-activity relationships (SAR) for nine marketed GnRH analogs like triptorelin and nafarelin, mapping out potential pathways for enhanced drug design.
A particularly noteworthy discovery involved the modification of a glycine at position 6 with D-amino acids (such as D-Trp), which improved receptor binding and activity while minimizing the effects of mutations in the binding pocket—an exciting advancement for future peptide drug development.
This groundbreaking study not only sheds light on the complexities of the GnRH-GnRHR relationship but also lays a vital foundation for innovative drug designs targeting reproductive disorders and cancers. The secrets of GnRH are now being unlocked, offering hope for countless patients.





 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)