Unlocking the Secrets of Protein Folding: A Legacy in Fighting Neurodegenerative Diseases
2025-06-18
Author: Yu
The Mystery of Protein Folding Revealed
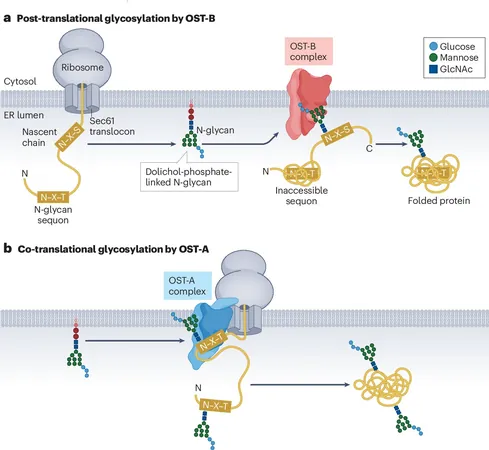
Protein folding is one of biology's greatest enigmas, vital for our bodies' proper function. At the heart of this intricate process lies a special code known as the 'glyco-code,' which plays a pivotal role in how these complex molecules assume their necessary shapes.
A Lifelong Quest by Daniel Hebert
The late Daniel Hebert, a revered professor at the University of Massachusetts Amherst, dedicated his life to unraveling this mystery. Shortly before his passing, he and his co-authors produced a groundbreaking review published in *Nature Reviews Molecular Cell Biology,* showcasing everything we know about the glyco-code.
Beyond DNA: The Hidden Codes of Life
For decades, it was believed that DNA was the sole arbiter of life. However, recent discoveries have revealed that intricate processes occur within the endoplasmic reticulum (ER), the cell's protein factory, where approximately one-third of all human proteins are matured. This secreted protein ensemble, the 'secretome,' orchestrates crucial bodily functions, from immune responses to digestion.
The Role of Chaperones in Protein Quality Control
Chaperones are molecular helpers that ensure proteins fold correctly. They identify incorrectly folded proteins, giving them a chance to refold or marking them for destruction if they fail. Yet, when this chaperone system malfunctions, the consequences can be dire, potentially leading to devastating diseases like Alzheimer's, emphysema, and cystic fibrosis.
Decoding the Glyco-Code: How Chaperones Work Their Magic
Kevin Guay, Hebert's final graduate student, emphasizes the chaotic environment of the ER, where carbohydrate-related chaperones guide the folding process. Chaperones rely on sugar molecules, specifically N-glycans, which act like postal codes, guiding proteins to their destinations.
A Paradigm Shift in Understanding Protein Maturation
Guay's previous research highlighted how the enzyme UGGT tags misfolded proteins using N-glycans, allowing chaperones to correct or eliminate them. Their latest comprehensive review delineates the mechanisms by which N-glycans are attached, their role in directing proteins, and the journey of correctly folded proteins as they exit the ER.
A Call to Action: Advancing Research for Better Treatments
Lila Gierasch, a distinguished professor and long-time collaborator of Hebert, stresses the significance of understanding the chaperone system thoroughly. As N-glycan placement emerges as crucial for protein maturation, advancing this research could pave the way for innovative treatments targeting diseases stemming from protein misfolding.
Hebert's lifetime of work has set the stage for future discoveries that may ultimately transform how we combat neurodegenerative diseases, offering hope for patients and families affected by these conditions.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)