
Unlocking the Secrets of Inherited Disorders: How G-Protein Mutations Could Pave the Way for Tailored Treatments
2025-05-21
Author: Jia
Revolutionary Insights into Rare Genetic Disorders
Groundbreaking research has shed light on how specific mutations in a G protein can profoundly impact individuals suffering from a rare and maternally inherited disorder, potentially paving the way for more targeted therapies.
Spotlighting Gαs Mutations
The study reveals exciting possibilities for customizing treatment plans for conditions linked to the Gαs subunit (GNAS), depending on the unique mutations present in each patient.
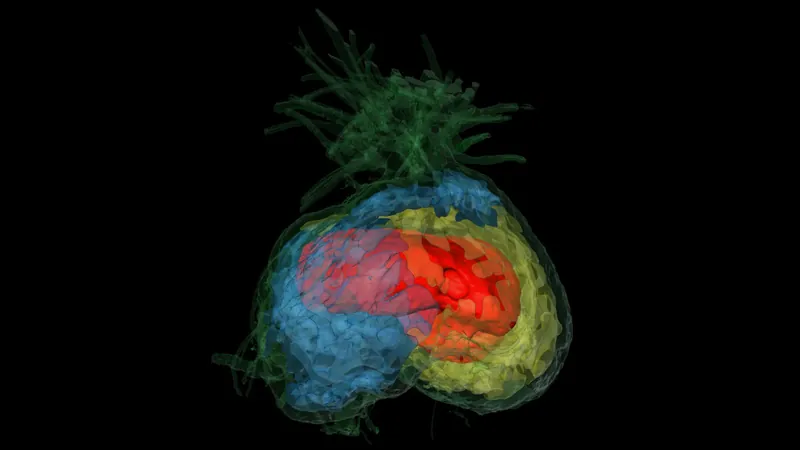
A Deeper Dive into GPCRs and Their Role
Published in the journal Science Signaling, these findings are critical not just for understanding pseudohypoparathyroidism, specifically type Ic (PHPIc), but also for the broader implications concerning G protein-coupled receptors (GPCRs). These essential receptors play a vital role in numerous bodily functions including metabolism, vision, and neurotransmission.
Understanding Pseudohypoparathyroidism Type Ic
PHPIc, inherited through maternal lines, leads to resistance against parathyroid hormone and presents symptoms like low calcium levels, high phosphate levels, and elevated parathyroid hormones. This condition encompasses a range of traits known as Albright’s hereditary osteodystrophy, including stunted growth, a round face, and shorter bones.
What Causes PHPIc?
This autosomal dominant disorder arises from loss-of-function mutations in the GNAS gene, which is activated by GPCRs. A team led by Theo Redfern-Nichols, PhD, from the University of Cambridge, investigated two specific Gαs variants, L388R and E392K, to see how they influence GPCR signaling in the kidneys.
The Impact of Gαs Variants Revealed
Despite the hundreds of GPCRs available, only 16 Gα proteins are available to couple with them, meaning that mutations in a single G protein can affect multiple receptors. The researchers identified GPCRs impacted by these mutations and utilized advanced 3D modeling, cellular analyses, and transcriptomics to demonstrate the effects on Gαs activation.
Mutations with Unique Effects
The results were striking: L388R obstructed the interaction between the Gαs subunit and GPCRs, while E392K inhibited GPCRs from activating the Gαs complex. This suggests that each unique mutation alters distinct stages of Gαs activation, opening doors to personalized treatment strategies based on a patient's genetic makeup.
A Roadmap for Future Research
These findings also lay the groundwork for exploring GPCR-G protein interactions in various tissues under both normal and pathological conditions. In the researchers' words, "Identifying individual causal mutations in specific patients could be crucial for guiding therapeutic strategies and enhancing the likelihood of successful outcomes." This could change the future of treatment for countless individuals affected by genetic disorders.

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)