Unlocking the Secrets of an 80-Year-Old Mystery: The Crystal Structure of TBAB Hydrate Revealed!
2025-07-18
Author: Ming
A Breakthrough in Science After Eight Decades!
For 80 years, scientists have been puzzled by the crystal structure of tetra-n-butylammonium bromide (TBAB) hydrate, known as TBAB·26H2O. But thanks to recent advancements, this longstanding mystery has finally been unraveled!
The Importance of TBAB Hydrate
Discovered in 1940, TBAB hydrate has become a cornerstone of various industrial applications, particularly in air conditioning systems. Gaining insight into its crystal structure will enable researchers and engineers to optimize its use even further, making this breakthrough especially significant.
The Cutting-Edge Research
A study, published on July 17 in *Crystal Growth & Design*, highlights how researchers, led by Sanehiro Muromachi from Yokohama National University, utilized synchrotron radiation to decode the elusive structure of TBAB hydrate. "For decades, the ambiguity surrounding its structure inhibited scientific understanding and practical application," stated Muromachi.
How It Works: The Science Behind the Structure
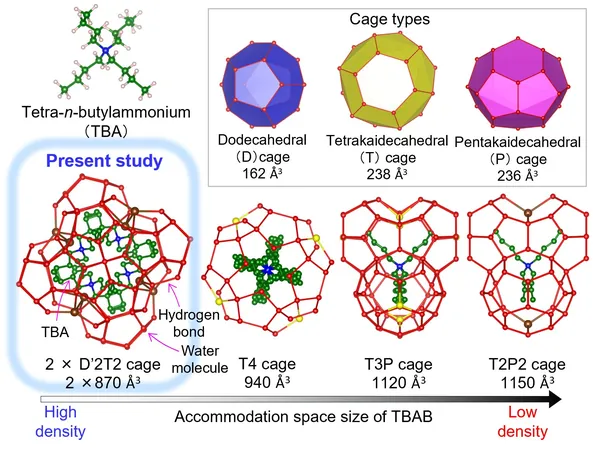
Inspired by the unique hydrogen-bonding properties of water, TBAB·26H2O is a semiclathrate hydrate, composed of TBAB molecules encased in a water molecular cage. This capability makes it a prime candidate for energy storage, especially in temperature-sensitive applications.
Revealing the Hidden Features
Using Japan’s Super Photon Ring-8 (SPring-8), researchers discovered that the TBAB hydrate features a Jeffrey's type III hydrate structure, presenting distinct attributes that contribute to its density and efficiency. This advancement in understanding paves the way for improved thermal storage capabilities.
Implications for the Future
With this newfound knowledge, the research team aims to explore innovative applications in energy-efficient technologies such as air conditioning, gas separation, and carbon capture. Muromachi mentioned, "We plan to extend these insights to other hydrate-forming systems, opening countless possibilities!"
A Collective Effort
The research was enriched by contributions from various experts, including Nobuhiro Yasuda and Hiroyasu Masunaga from the Japan Synchrotron Radiation Research Institute, and Takeshi Sugahara from Osaka University.
The Adventure Continues!
This breakthrough not only demystifies an important material but also sets the stage for future innovations in the realm of water-based functional materials, promising a cleaner, more sustainable future.
 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)