Unlocking the Power of Water: Transforming Carbon Waste into Valuable Fuels
2025-07-30
Author: Mei
The Innovative Breakthrough in Carbon Conversion
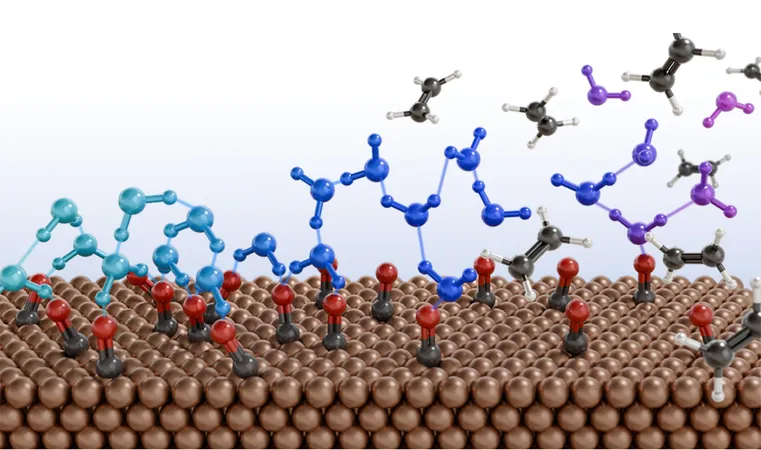
In a groundbreaking discovery, materials scientist Shoji Hall and his team at the University of Pennsylvania have uncovered how modifying the surface of water can enable the sustainable conversion of carbon monoxide into high-energy fuel products like ethylene.
Tackling Climate Change with Carbon Recycling
As carbon monoxide (CO) and carbon dioxide (CO₂) levels continue to rise, threatening our planet's climate and ecosystems, researchers are urgently seeking methods to recycle these pollutants into cleaner energy sources. The multi-carbon molecule ethylene (C₂H₄) shines as a promising solution: it can release stored energy when combusted or serve as a foundational building block for a range of products, from packaging materials to pharmaceuticals.
The Challenge of Carbon Conversion
Converting CO and CO₂ into valuable compounds like C₂H₄ presents a significant hurdle; even well-known catalysts like copper can generate unwanted byproducts. Hall's team has identified a surprising partner in this endeavor: water.
Harnessing Water's Unique Properties
Published in Nature Chemistry, the research reveals that by adjusting the concentration of sodium perchlorate (NaClO₄) in water, the typically orderly hydrogen bonding network of water molecules can be disrupted at the metal interface. This disruption is crucial for electrochemical catalysis, where electricity, water, and metal surfaces collectively drive the conversion of CO into multi-carbons.
"This chaotic arrangement of water molecules is the key to facilitating the bonding of carbon atoms, a process that has long impeded our ability to produce ethylene from CO," states Hall. This disordered environment acts like a microscopic spiderweb, enabling carbon atoms to bond more easily.
Revolutionizing Chemical Reactions with Simplicity
What excites Hall the most is the simplicity of the solution. By fine-tuning ordinary water, we can recycle harmful gases like CO and CO₂ into valuable fuels, sidestepping the need for costly and complex solvents.
Experimental Success: A Leap Forward in Efficiency
To validate their theory, Hall's lab conducted electrochemical reactions on copper-coated electrodes submerged in a salty water solution containing CO. By incrementally increasing NaClO₄ levels, they measured the efficiency of CO conversion to various products, particularly ethylene.
As they increased the concentration of NaClO₄ from 0.01 to 10 molal, they discovered a striking increase in Faradaic efficiency—meaning that a higher percentage of electrons contributed to desired product formation—soaring from 19% to 91%. Remarkably, hydrogen gas, a typical unwanted byproduct, nearly vanished, and ethylene production skyrocketed eighteenfold.
Entropy: The Driving Force of Reaction Speed
Exploring the role of proponents in the reactions, the researchers experimented with heavy water (D₂O) to assess proton transfer effects. Contrary to expectations, it was the entropy—or the increasing disorder among water molecules—that boosted reaction efficiency, rather than the movement of protons. This discovery suggests a novel approach to controlling surface chemistry.
Implications Beyond Carbon Conversion
Beyond the technical success, this research carries vast implications: water, a common component in electrochemical systems, could be strategically utilized to enhance a variety of chemical reactions. Hall emphasizes, "Electrochemistry is rife with hidden mechanisms; we believe that the interfacial structure of water is among the most influential. If we view water as a co-designer—not merely a solvent—we can significantly improve reaction outcomes."
Future Prospects: Expanding the Horizons of Chemical Engineering
Looking forward, Hall's lab aims to apply their findings to more complex reactions, such as combining carbon sources with nitrogen to create fertilizer precursors. Ultimately, this innovative approach could pave the way for precise engineering of chemical transformations, potentially revolutionizing how we manage carbon waste.



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)