Unlocking the Glow: Scientists Master Cerium's Luminescence
2025-04-23
Author: Rajesh
Revolutionary Breakthrough in Rare Earth Element Luminescence
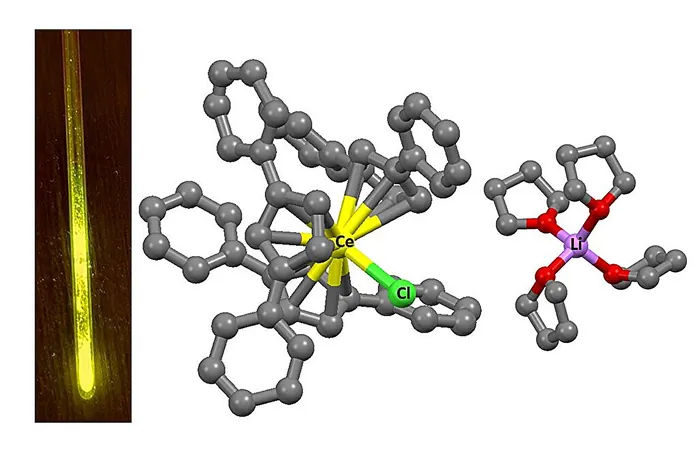
In a groundbreaking discovery, researchers from HSE University and the Institute of Petrochemical Synthesis of the Russian Academy of Sciences have unlocked the secrets of rare earth element luminescence, revealing how to manipulate both color and brightness. Typically, cerium emits ultraviolet light, but thanks to this pioneering work, it can now shine with a brilliant yellow glow!
A New Era for Light Sources and Displays
This breakthrough could pave the way for innovative applications in lighting technology, including advanced light sources, cutting-edge displays, and high-performance lasers. The research findings were recently published in the journal *Optical Materials*.
How Luminescence Works in Rare Earth Elements
Rare earth elements are crucial in microelectronics and LEDs due to their unique ability to emit light in precise colors. This phenomenon stems from the way electrons behave during energy absorption and release. When atoms absorb energy (from light or electricity), electrons jump to higher energy levels. Soon after, they return to their original state, emitting light in the process—a phenomenon known as luminescence.
Changing the Game with Chemical Environments
Historically, the color of luminescence from these elements has been fixed due to their stable electron transitions between 4f orbitals. Scientists found that by modifying the surrounding chemical environment of cerium, praseodymium, and terbium, they could alter this color dramatically.
Using organic ligands that encircle the metal ions, the researchers created geometrically tailored complexes that produced a specific electrostatic field around the ion. This unique environment modified the energy levels of the 5d orbitals, effectively shifting cerium's light emission from the ultraviolet spectrum into the red range, reaching wavelengths of up to 655 nanometers.
A Collaborative Effort Unveils the Mechanism
Daniil Bardonov, a master's student involved in the research, stated, "Although color changes in luminescence had been observed before, we didn't fully understand the mechanisms behind them. Our collaboration with physicists has enabled us to delineate these processes clearly, allowing us to design compounds with unique electronic structures."
New Pathways for Energy Transfer
In conventional setups, energy transfers directly to the 4f electrons. However, the team discovered a new mechanism where the energy is first directed to an intermediate 5d state before being transferred to the 4f electrons, refining how luminescence occurs.
The Future of Customized Luminescence
This research not only sheds light on how atomic environment influences luminescence but also streamlines the design of materials for tailored optical properties. Fyodor Chernenkiy, another contributor to the study, remarked, "Knowing how to precisely tune luminescence allows us to select compound structures intentionally, paving the way for materials with exceptional properties."
As this research continues to unfold, we may soon see a shift in how we harness light, making ordinary objects glow in new and unexpected ways!



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)