Unlocking The Future of Recombinant Protein Production with Tobacco Plants: New Strategies Revealed!
2024-09-25
Introduction to Plant Molecular Farming (PMF)
In the realm of biotechnology, Plant Molecular Farming (PMF) stands out as a groundbreaking approach, leveraging the natural biosynthetic capabilities of plants to produce a wide array of recombinant proteins. These include vital industrial and therapeutic enzymes, making PMF a formidable alternative to conventional methods like microbial fermentation and mammalian cell culture. The advantages are significant: PMF promises lower production costs, high yields, and eliminates risks associated with human pathogens and endotoxins. Additionally, the versatility of plants allows for tailored production of proteins to meet specific demands.
Tobacco as a Prime Candidate for PMF
Among the plant species, tobacco—specifically Nicotiana benthamiana and Nicotiana tabacum—has garnered attention for its unique traits. The plants’ relatively weak basal immunity and minimal ribonucleic acid (RNA) silencing mechanisms, which typically degrade foreign RNA, position them as prime candidates for efficient recombinant protein production. Their rapid growth cycle and substantial biomass further enhance their appeal for this purpose.
Navigating Limitations in Protein Production
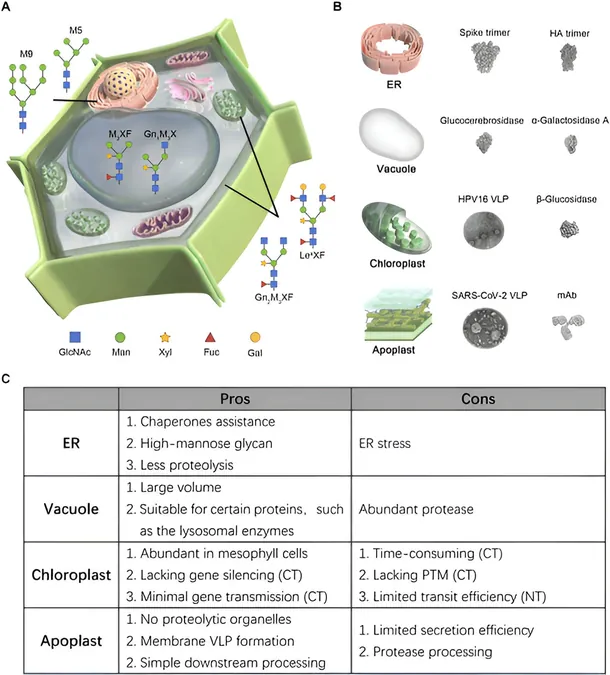
Nevertheless, harnessing these benefits effectively requires navigating certain limitations. One significant aspect is the need for engineering tobacco cells to ensure the precise targeting of recombinant proteins to specific subcellular locations. A comprehensive review recently published in BioDesign Research delves into various strategies aimed at optimizing subcellular localization. The study highlights key targeting pathways that direct recombinant proteins to four major compartments: endoplasmic reticulum (ER), vacuole, chloroplast, and apoplast.
Optimizing Subcellular Localization
"Optimizing subcellular localization for individual target proteins is crucial for successful protein synthesis and utilization in the pharmacological industry," emphasizes Dr. Shi-Jian Song from the Chinese Academy of Agricultural Sciences. Among the compartments, the ER stands out as a critical area for protein localization due to its role in protein-folding, facilitated by molecular chaperones, thereby reducing degradation risks. Proteins directed to the ER undergo glycosylation—an essential modification for many therapeutic proteins—which can be achieved using specific signal peptides and retention sequences designed for ER targeting.
Challenges with ER Protein Production
However, Dr. Inhwan Hwang, co-author of the study, points out a significant caveat: "Overloading the ER might lead to stress and significantly reduce protein yields." Thus, managing expression levels carefully is vital to avoid these pitfalls.
The Role of Vacuoles in Protein Storage
The study also uncovers the potential of the plant vacuole, which occupies a significant volume in tobacco leaves, as an efficient storage space for recombinant proteins. The trafficking routes to vacuoles are diverse; strategic engineering can facilitate direct transport from the ER, bypassing the Golgi apparatus. Yet caution is warranted, as some proteins may degrade in vacuolar environments due to the presence of proteolytic enzymes. It’s best to target proteins that can endure acidic conditions akin to the human lysosome.
Exploring the Chloroplasts for High Protein Content
Chloroplasts are another focal point, being centers for high native protein content. Two pivotal methods for recombinant protein accumulation here are chloroplast transformation and nuclear transformation. While chloroplast transformation offers stable gene expression, the challenge of creating high-yield plants through this method is notable. Conversely, nuclear transformation, involving a fusion with chloroplast transit peptides, permits quicker protein production, making it preferable for certain proteins that do not demand complex biochemical modifications.
Utilizing the Apoplast for Protein Production
The apoplast, located between the cell membrane and the wall, emerges as a prime location for protein accumulation. It allows for simplified purification processes, although larger protein complexes may necessitate conventional purification methods. To counteract structural integrity issues from proteases in the apoplast, co-expression of protease inhibitors is being used as an emerging technological strategy.
Challenges and Future Outlook
PMF holds immense potential to transform the landscape of recombinant protein production. Yet, various hurdles remain, such as standardization of production levels, maintaining quality, and addressing cost issues. Dr. Song articulately states the need for strict adherence to safety protocols, increased public engagement, and transparent practices to ensure the normalized use of transgenic plants in industry.
Conclusion
Ultimately, by optimizing the tobacco plant chassis, effectively allocating resources, and developing a toxin-free environment for production, significant advancements in PMF are on the horizon. The commercialization of biomanufacturing in this domain represents a pivotal milestone, echoing the promise and progress of modern biotechnology. As researchers continue to unlock the potential of tobacco for protein production, the future looks bright for medical and industrial applications alike! 🌱✨


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)