Unlocking Nature's Defense: How Calprotectin Starves Bacteria of Essential Zinc
2025-09-22
Author: Daniel
In the ongoing battle against bacterial infections, our immune system employs a clever tactic: withholding zinc, a crucial element for bacterial survival. Central to this defense is calprotectin, a protein abundant at infection sites that binds transition metals like zinc with exceptional strength. Recent research has finally revealed the exact structure of zinc when it binds to calprotectin, shedding light on how this protein effectively starves harmful microbes.
The Breakthrough: First Zinc-Bound Structure of Calprotectin
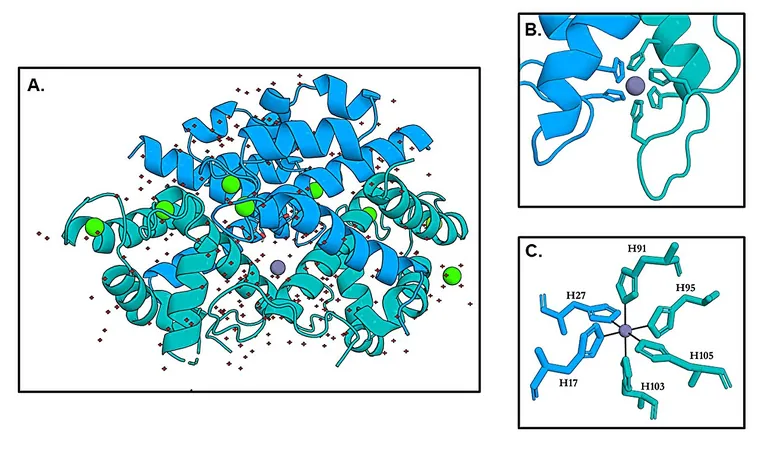
Researchers have unveiled the first-ever zinc-bound crystal structures of calprotectin, providing unprecedented insight into its binding mechanism. At a specific site known as His6, zinc is encased in an octahedral formation formed by six histidines from the calprotectin subunits S100A8 and S100A9, including two from the flexible tail of S100A9.
Unraveling the Mystery of Zinc Coordination
Calprotectin's ability to hold onto zinc is not just about structure; it's about strength. Even when alterations are made to the tail of the protein, calprotectin maintains a remarkable affinity for zinc at picomolar levels. This high affinity showcases that the protein's metal-keeping capabilities are robust, regardless of structural changes.
Implications for Pathogen Growth
The research also focuses on a major pathogen, Staphylococcus aureus, revealing that calprotectin significantly inhibits its growth. Disabling the metal-binding sites nearly nullifies this antibacterial effect, reinforcing the idea that sequestering metals is fundamental to calprotectin's antimicrobial role. But there's more: manipulating the tail of the protein alters not only how biomass grows but also how calprotectin interacts with bacteria.
The Two-Pronged Attack of Calprotectin
The findings draw two critical conclusions. First, the chemistry of calprotectin is clarified—zinc is tightly locked away in a six-histidine cage, while at another binding site, it adopts a four-coordinate geometry. Second, the biology of this protein reveals it operates on two levels: it effectively withholds zinc while also playing an active role in shaping bacterial interactions and community structures.
Why Understanding Calprotectin Matters
The implications are profound. Biofilms created by S. aureus are notoriously difficult to treat, complicating wound care and increasing the risk of infections. By deciphering how calprotectin both hoards zinc and influences bacterial communities, researchers uncover new potential strategies for combatting these resilient biofilms.
Future Directions in Treatment Strategies
On a practical level, the design of histidine-rich cages offers a roadmap for creating zinc-immobilizing therapies. Moreover, enhancing calprotectin's natural interactions could complement existing antibiotics and steer microbial cultures away from forming stubborn, adherent communities.
A New Era for Calprotectin Research
For enthusiasts of immunology, these discoveries are groundbreaking: the first zinc-bound structures of calprotectin, concrete proof of how zinc is coordinated at its binding sites, and critical insights into the relationship between this protein and pathogenic bacteria. Together, these findings illuminate both the chemistry and biology behind zinc withholding, marking a significant advancement in our understanding of innate immunity.
As researchers like Dr. Yasiru Randika Perera continue to explore these mechanisms, the fight against infections becomes not just a medical challenge, but a deeply intricate dance between host and pathogen.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)