Unlocking Life's Secrets: The Lipocone Superfamily and Its Surprising Evolution
2025-09-11
Author: Daniel
Unearthing a Hidden Protein Superfamily
In a groundbreaking study, researchers have revealed a fascinating set of previously unknown enzymatic domains known as the Lipocone superfamily. This discovery traces their evolution from bacterial defense mechanisms to crucial components of human development, providing deep insights into the origins and functions of these significant molecules across multiple biological kingdoms.
Revolutionary Insights into Wnt Proteins
Among the superfamily’s key players is the Wnt protein, integral to animal and human developmental processes, and linked to various diseases, including cancers. While Wnt has been known for over four decades, its evolutionary backstory remained largely obscured until recently.
Co-lead author A. Maxwell Burroughs, a Staff Scientist at the National Institutes of Health (NIH), highlights a pivotal moment in 2020 when they discovered bacterial versions of Wnt, resembling toxins used in biological warfare against viruses. This spurred their quest to map out the evolutionary lineage and potential functions of these proteins.
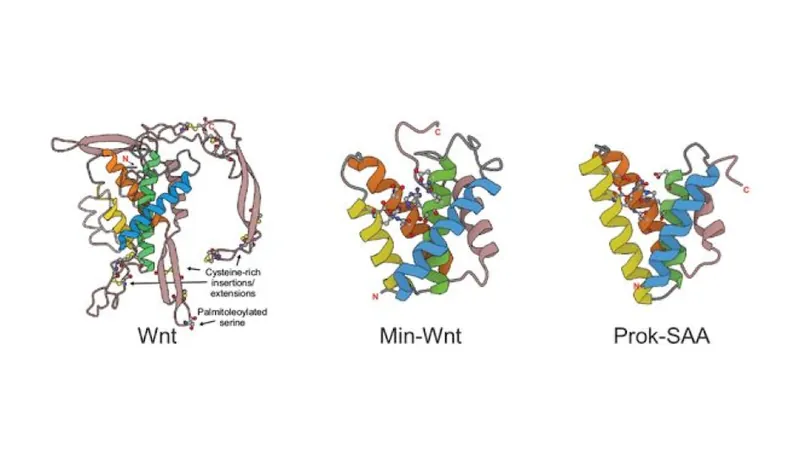
A Molecular Marvel: The Lipocone's Unique Structure
The team uncovered a distinct alpha-helical core structure shared by both bacterial and metazoan Wnt, leading them to conduct comprehensive sequence-based searches. Ultimately, they identified a remarkable network of 30 families, with 17 previously undefined functionally, all uniting under the Lipocone superfamily.
Structural Analysis Unveils Lipocone Functionality
Using AlphaFold, an AI-driven structural biology tool, the researchers predicted the three-dimensional shapes of these proteins from their amino acid sequences. They deduced that the Lipocone's structure allows for lipid interaction, suggesting a sophisticated mechanism for manipulating phosphate-linked chemical groups, vital for cellular functions.
Tracing Evolution: From Bacteria to Complexity
The team's evolutionary reconstruction indicated diverse roles for the Lipocone superfamily in modifying lipid structures within various membranes and producing key cell-structure molecules like peptidoglycan and lipopolysaccharides. Their analysis revealed significant involvement in processes such as bacterial conflict, stress responses, lipid metabolism, and even antibiotic resistance.
Wnt's Evolutionary Shift: From Enzyme to Communicator
One intriguing finding was the evolutionary transformation of certain families from hydrophobic integral membrane proteins to soluble effector molecules. This shift is exemplified by Wnt, which, despite losing its enzymatic activity, maintains an ancient role in cell communication. Co-lead author Gianlucca Nicastro speculates that Wnt's binding capabilities may enable it to interact with other molecular players, such as lipids, in ways not yet understood.
A Significant Step Forward in Lipid Biochemistry
Senior author L. Aravind, a key figure at the NIH, emphasizes that their research marks a milestone in identifying a large, unexplored protein superfamily, predicting various catalytic activities for proteins that have puzzled scientists for years. This work not only illuminates the origins of Wnt signaling but also offers fresh perspectives on immunity and the complex interactions across the Tree of Life.
Conclusion: A New Chapter in Protein Evolution Research
Published today in eLife, this monumental study sheds light on the Lipocone superfamily, paving the way for future discoveries in lipid metabolism and its implications in health and disease. It's an exciting time in the field of protein evolution, as researchers continue to unravel the intricate web of life at a molecular level.



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)