Unlocking Clean Energy: The Game-Changing Role of Crystal Shape in Cuprous Oxide Catalysts
2025-04-18
Author: Yu
Can Crystal Shape Revolutionize Clean Energy?
Think the shape of a crystal couldn’t impact its efficiency in clean energy technology? Think again! A groundbreaking study unveils how the geometric form of cuprous oxide (Cu₂O) crystals plays a pivotal role in their effectiveness as catalysts.
A Collaborative Breakthrough in Energy Research
Conducted by an innovative team from National Taiwan University, National Tsing Hua University, and National Yang Ming Chiao Tung University, this research highlights the importance of crystal facets in oxygen reduction reactions (ORR). This reaction is crucial for fuel cells, which convert chemical energy into electricity. While platinum is the go-to material, its high price and limited availability make Cu₂O an appealing alternative—if harnessed correctly.
Three Shapes, Unique Properties
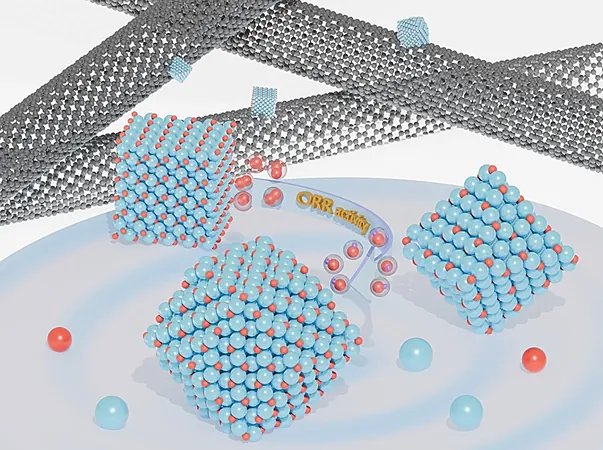
In their pursuit of efficiency, the researchers crafted Cu₂O crystals into three distinct shapes: cubes, octahedra, and rhombic dodecahedra. Each shape unveils different crystal facets—{100}, {111}, and {110}—and was enhanced with carbon nanotubes to boost conductivity.
The Surprising Star: Rhombic Dodecahedron
The findings revealed that the rhombic dodecahedron, exposing the {110} surface, exhibited unparalleled catalytic activity for ORR. Meanwhile, the cube shape demonstrated impressive stability. This duality emphasizes the need for a balance between reactivity and durability in catalyst design.
Crystalline Chemistry: A Deep Dive
Utilizing advanced quantum simulations alongside experimental lab work, the researchers discovered that the behavior of oxygen molecules drastically differs based on crystal surface interaction. The {110} surface, showing a weaker bond with oxygen, allows reactions to flow more effortlessly, corroborating their theoretical predictions with actual findings.
A Double-Edged Sword: Performance vs. Longevity
While the rhombic dodecahedron showcased stellar performance, its speed came with a price: faster degradation during operation, likely due to self-oxidation. On the flip side, the cube-shaped crystals, though less reactive, boasted remarkable longevity.
A Pathway to Next-Gen Catalysts
This pioneering research not only elucidates the performance disparities across different facets but also opens the door to the future of low-cost catalyst design through meticulous control of crystal shapes. Prof. Jyh-Pin Chou of National Taiwan University emphasizes, “By fine-tuning the geometry of crystal surfaces, we can tailor their reactivity and stability, which is crucial for advancing sustainable energy technologies.” This could be a monumental leap towards a greener, more efficient energy future!



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)