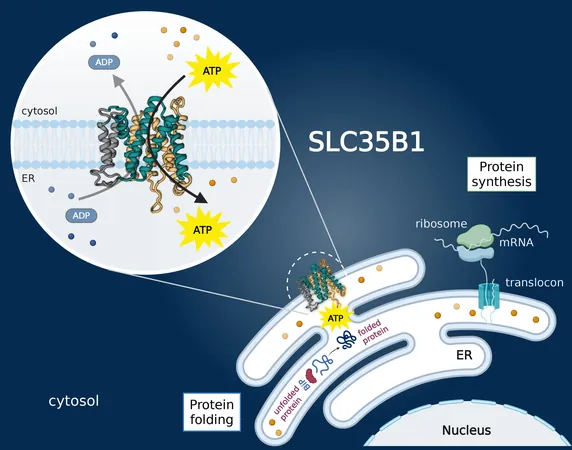
Unlocking Cellular Energy: How a Key Protein Transports ATP into the Endoplasmic Reticulum
2025-05-21
Author: Arjun
A Breakthrough in Cell Biology!
Scientists have finally cracked a long-standing mystery in cell biology by revealing the mechanism behind how ATP—the cell's primary energy currency—is transported into the endoplasmic reticulum (ER). This discovery is crucial, as imbalanced energy transport is linked to severe conditions such as type 2 diabetes, cancer, and neurodegenerative diseases.
Meet the Transporter: SLC35B1!
In a groundbreaking study published in Nature, researchers identified the SLC35B1 protein as the essential gateway for ATP's entry into the ER. Led by David Drew, a biochemistry professor at Stockholm University and SciLifeLab, the team provided unprecedented structural details about how ATP is delivered to the ER—a cellular hub responsible for packaging proteins and lipids.
Energy Delivery: A Game Changer!
"For decades, we’ve grappled with the question of how ATP reaches the ER, and today we have an answer! By confirming SLC35B1 as the critical transporter and elucidating its structure using cryo-electron microscopy, we not only clarify a key biological process but also pave the way for innovative therapeutic strategies," stated Drew.
Implications for Drug Development!
The ramifications of this research are profound. As disrupted ER function is implicated in various diseases marked by protein misfolding and ER stress, targeting SLC35B1 could open new avenues for drug development. "Understanding how energy is delivered into the ER allows us to think creatively about addressing diseases linked with ER dysfunction. Fine-tuning SLC35B1's activity might become a revolutionary approach in restoring cellular balance in diseased states," added Drew.
Groundbreaking Findings from Collaborative Research!
Although SLC35B1 had previously been suspected of acting as an ATP transporter, this study finally provides biochemical and structural validation. Collaborating with the Giulio Superti-Furga Lab in Austria, a large-scale CRISPR/Cas9 knockout screen confirmed SLC35B1 as one of the five most crucial transporters for cellular growth.
Innovative Imaging Techniques!
To visualize this important protein, Norimichi Nomura's team from Kyoto Medical School developed an antibody against human SLC35B1, enabling the research team to increase the protein's size for imaging. Using advanced cryo-electron microscopy at SciLifeLab, Drew’s team captured multiple conformations of SLC35B1, shedding light on its mechanisms for ATP transport into the ER.
Why This Matters!
With the structural revelations highlighting critical amino acids involved in ATP binding, researchers now have exciting potential targets for future therapeutic interventions, especially in the context of diseases where ER dysfunction plays a central role. This breakthrough not only enhances scientific understanding but also ignites hope for innovative treatments against some of today's most pressing health challenges.

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)