Unleashing the Power of AI: Bridging the Gap Between Animal and Human Disease Research
2024-09-25
Unleashing the Power of AI: Bridging the Gap Between Animal and Human Disease Research
In the realm of medical research, the leap from animal models to humans is crucial for understanding disease mechanisms and refining targeted therapeutic strategies. The advent of sophisticated techniques like single-cell RNA sequencing has revolutionized our ability to explore the molecular and cellular parallels between humans and their animal counterparts with extraordinary precision. Yet, current methodologies for a meaningful comparison of this invaluable data are still limited.
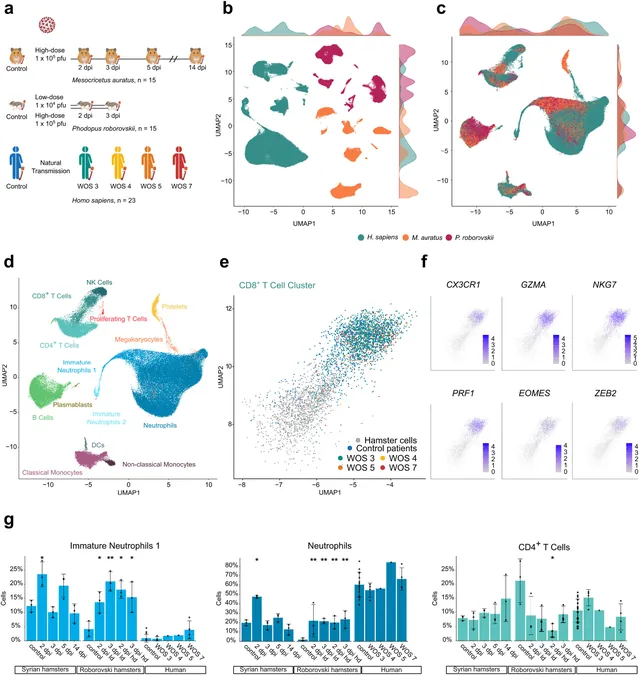
A groundbreaking study recently published in eBioMedicine highlights how artificial intelligence (AI) is forging a new path in this field. Researchers from Leipzig University’s Institute for Medical Informatics, Statistics and Epidemiology (IMISE) and the ScaDS.AI initiative, in collaboration with the Department of Respiratory Medicine and Critical Care Medicine at Charité, have developed a state-of-the-art AI model targeting COVID-19. This model utilizes blood data from humans and various hamsters, analyzing conditions ranging from moderate to severe COVID-19 at a molecular level.
Dr. Holger Kirsten, a leading scientist from IMISE, emphasized the transformative potential of their work: “We've demonstrated that employing robust deep learning models in combination with biologically informed analyses can significantly narrow the translational gap between animal and human patient data. Our AI can systematically decipher the molecular distinctions between species and translate these insights into relevant patterns for humans, effectively 'humanizing' the valuable data derived from animal studies.”
The study found intriguing similarities in immune activation responses between Syrian hamsters and humans, particularly in cases of moderate COVID-19. Dr. Geraldine Nouailles, a Scientific Group Leader at Charité, noted that monocytes—the precursors to macrophages that play a vital role in immune defense—show remarkably similar activation profiles in both species.
However, when examining severe disease cases, the researchers identified that neutrophils from Roborovski hamsters offer the most comparable insights to human immune responses. “These rapid-reacting immune cells exhibit behavior strikingly akin to that seen in humans,” Dr. Nouailles added, highlighting the critical linkages that can be drawn between animal and human data, which aligns with observations made during the pandemic.
Beyond the confines of COVID-19 research, the implications of this innovative methodology are vast. Kirsten remarked on the broader applications of single-cell RNA sequencing: “This approach is exceptionally suited to uncover similarities and differences in the molecular and cellular makeup of animals and humans across a spectrum of diseases.”
The team’s future aspirations are equally ambitious. They plan to refine the AI-driven methodology for application to other animal models in studies assessing immunomodulatory therapies, such as CAR T cell therapy—a groundbreaking treatment for certain cancer types.
By harnessing the power of AI, scientists are charting a course toward more effective preclinical studies, ultimately improving the translation of findings from bench to bedside. As this technology evolves, we may find ourselves on the brink of unprecedented advancements in our understanding and treatment of human diseases. Stay tuned for more exhilarating updates from the frontlines of medical research!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)