Uncovering the Dark Side of ZIP7: How It Fuels Diabetic Cardiomyopathy by Blocking Mitophagy in Mice
2024-11-07
Author: Wei
The Research Behind the Findings
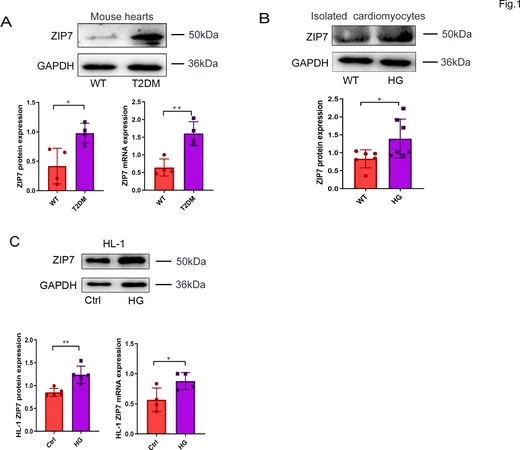
The scientists utilized cell cultures and genome editing techniques, involving the HL-1 and AC16 cell lines, to explore the role of ZIP7. Adult cardiomyocytes were isolated from C57BL/6J mice and were subjected to either normal or high glucose conditions. Interestingly, when cells were exposed to a high glucose environment, ZIP7 levels significantly increased, indicating that elevated glucose could trigger its expression.
To further analyze ZIP7's impact, researchers employed a cardiac-specific conditional knockout (cKO) model to study the function of ZIP7 in vivo. The results were striking: the absence of ZIP7 not only reduced mitochondrial reactive oxygen species (ROS) but also improved heart function and structure, suggesting that ZIP7 plays a crucial role in the oxidative stress and cardiac dysfunction seen in DCM.
A Vicious Cycle: ROS and Mitophagy
Elevated ROS generation has been implicated in the pathology of diabetic cardiomyopathy, and this study confirmed that T2DM mice produced significantly higher levels of mitochondrial ROS. Notably, the researchers found that knocking out ZIP7 reduced ROS levels, hinting at a direct link between ZIP7 and oxidative stress in diabetic hearts.
Mitophagy, a process responsible for removing dysfunctional mitochondria, was found to be inhibited in T2DM. This suppression leads to further ROS accumulation and mitochondrial damage, creating a vicious cycle that exacerbates heart disease. Enhanced ZIP7 levels were associated with decreased mitophagy markers, confirming that ZIP7 hampers the heart's ability to manage oxidative stress effectively.
The ZIP7 and PINK1-Parkin Connection
A critical focus of this study was the PINK1-Parkin pathway, a key regulator of mitophagy. The researchers discovered that T2DM reduced the levels of PINK1 and Parkin in mitochondrial fractions, an effect that was reversed by ZIP7 cKO. This suggests ZIP7 mediates the suppression of this vital pathway, further establishing its central role in DCM development.
The importance of zinc transport in mitochondria was highlighted, with findings showing that T2DM leads to a decrease in mitochondrial zinc levels, an effect modulated by ZIP7 activity. This zinc depletion was linked to increased mitochondrial membrane potential and hyperpolarization, detrimental conditions that inhibit mitophagy.
Mitophagy: The Heart's Lifesaver
In conclusion, the absence of ZIP7 not only spurred the mitophagy process but also improved overall cardiac function and structure in T2DM mice. Researchers assert that targeting ZIP7 could serve as a promising therapeutic approach to mitigate the negative effects of T2DM on cardiac health.
As mounting evidence continues to elucidate the links between ZIP7, oxidative stress, and heart disease, the path forward may hold new hope for patients suffering from diabetic cardiomyopathy. Ultimately, this research presents a clarion call to rethink how we approach treatment and prevention strategies for diabetic heart complications.
This revelation invites further exploration of ZIP7 inhibitors as potential drugs to enhance mitophagy and protect heart health in diabetic patients, paving the way for innovative therapies in the future.




 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)