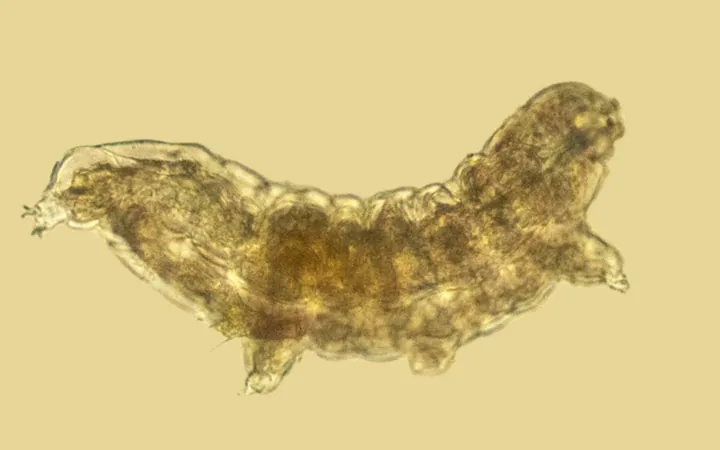
The Incredible Power of Water Bears: Enhancing Modern Technology through Ancient Biology
2024-12-22
Author: John Tan
Introduction
Microscopic marvels known as water bears, or tardigrades, are revolutionizing the way scientists capture high-resolution images of cellular structures. Renowned for their distinctive pudgy shape and resilience to extreme conditions, these ancient creatures hold clues to our understanding of the molecular building blocks of life—and they might just help solve some of today's pressing scientific challenges.
Tardigrades and Their Unique Adaptations
Tardigrades have ingeniously adapted to thrive in environments that would typically be fatal for most life forms. They possess specialized molecules called late embryogenesis abundant (LEA) proteins, which protect their cellular structures from damage. This remarkable ability allows them to enter a dormant state, surviving without water for years, only to rehydrate and regain their vitality decades later when conditions improve.
Breakthrough Study on Cryo-EM
A groundbreaking study led by Ci Ji Lim at the University of Wisconsin–Madison, recently published in *Nature Communications*, has revealed that these durable LEA proteins can significantly enhance the quality of images obtained through cryogenic electron microscopy (cryo-EM). This technique is essential for freeze-framing biological molecules to study their structures in a timely manner, yet it faces challenges due to biomolecule damage at the air-water interface of samples.
The Importance of Cryo-EM
Cryo-EM allows scientists to visualize proteins and other biomolecules at specific moments, crucial for understanding their functions and identifying new drug targets. However, the problematic interactions at the air-water juncture often result in proteins clumping or unfolding, leading to compromised data. Lim hypothesized that integrating LEA proteins into microscope samples could guard against these detrimental effects.
Experimental Findings
In their experiments, Lim and his team, including graduate student Kaitlyn Abe, tested LEA proteins on Polα-primase and other hard-to-image proteins like PRC2. The results were promising; the addition of LEA proteins provided a cost-effective and straightforward way to produce clearer, more reliable cryo-EM images without needing higher concentrations of the proteins being studied.
Expert Commentary
"This breakthrough offers a much-needed solution to a critical bottleneck in cryogenic imaging," Lim remarked. "The fact that these protective proteins evolved naturally to serve such a purpose in water bears is fascinating."
Future Implications
With this new methodology, researchers hope to develop a more nuanced understanding of protein structures, leading to significant advances in fields ranging from drug discovery to genetic engineering. Tim Grant, a fellow investigator at the Morgridge Institute for Research and collaborator on the study, has expressed optimism about the future of structural biology research using LEA proteins. “We’re starting to see biomolecules retain their integrity even at the air-water interface, paving the way for scientific revelations that were previously out of reach,” he added.
Conclusion
The use of LEA proteins could dramatically shift the landscape for cryo-EM research, potentially unlocking insights into a plethora of proteins that scientists have struggled to examine due to structural complexities. As Lim shared, “This new approach not only opens doors to previously unexplored protein structures but also represents an expansion of the tools researchers have at their disposal.”
In essence, the ancient water bear is rewriting the manual for modern technology, reminding us that nature can offer solutions to challenges we face today. With ongoing research into the protective capabilities of LEA proteins, we are on the brink of transformative discoveries that could reshape our understanding of biology. Stay tuned—this story is just beginning!



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)