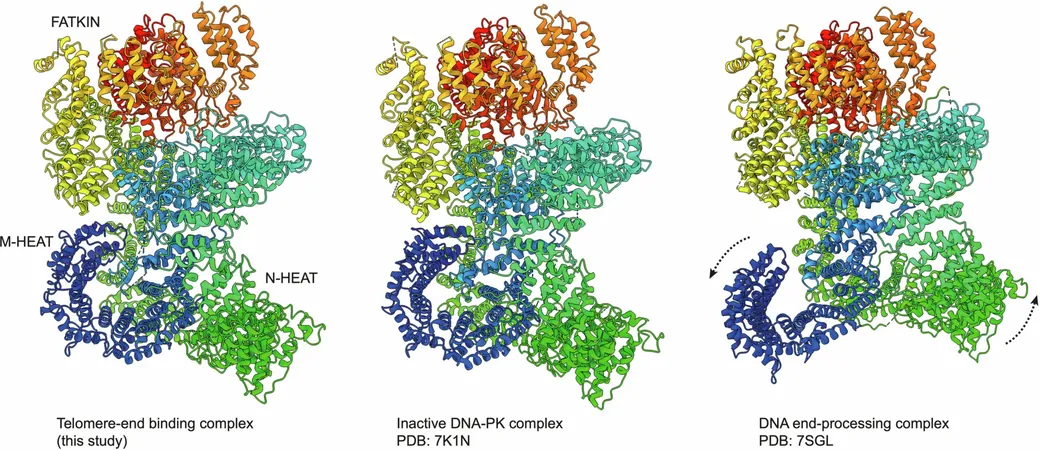
The Chromosome Protector: How Two Key Proteins Prevent Fatal Fusions
2025-05-06
Author: Daniel
A Breakthrough Discovery in Chromosome Defense
Researchers at the prestigious Institute of Cancer Research in London and Linköping University in Sweden have unveiled a groundbreaking mechanism that prevents chromosomes from accidentally merging, a potentially disastrous error for cells.
The Dynamic Duo: TRF2 and RAP1
At the heart of this discovery are two vital proteins—TRF2 and RAP1. Their partnership acts as a formidable barrier, blocking cellular repair processes that could misinterpret the natural ends of chromosomes as broken and in need of fixing.
Understanding Telomeres: The Chromosomal Caps
Each chromosome is capped with telomeres, serving as protective shields akin to the plastic tips on shoelaces. These caps are crucial for helping cells recognize healthy chromosome ends versus actual DNA damage.
The Role of DNA-PK: A Double-Edged Sword
When DNA incurs breaks elsewhere in the genome, a repair process known as non-homologous end joining (NHEJ) is activated. A key player in this process, DNA-PK, is responsible for drawing additional repair proteins to finalize the rejoining. Yet, at telomeres, this same repair mechanism must be kept at bay to avoid catastrophic chromosome fusions.
TRF2's Cloaking Device
Past studies have already showcased TRF2’s ability to create a protective loop at the terminal end of chromosomes, effectively rendering telomeres 'invisible' to the repair machinery. Another protein, Apollo, enhances this disguise, ensuring telomeres aren't misidentified as damaged DNA. This reveals an intricate network of protection that safeguards chromosome integrity.
RAP1: The Mammalian Gatekeeper
The spotlight then shines on RAP1, a protein with established roles in various species but whose function in mammals had remained a mystery. Past knowledge suggested RAP1 inhibits end-joining processes in yeast. Could it fulfill a similar role in human cells?
Signals of Danger: Fusions When Proteins Are Absent
In an insightful study published in Nature, scientists embarked on a quest to unravel RAP1's role by removing the genes for RAP1 and Apollo in mouse and human cells. The results were alarming, revealing that when both proteins were absent, around 15% of telomeres in mouse cells and a staggering 30% in human cells were fused. This showcased DNA-PK's errant activation at chromosome ends.
Blocking the Repair Brigade: RAP1's Unique Interaction
Through advanced imaging techniques, researchers demonstrated how RAP1 physically hinders DNA-PK from recruiting LIG4, another necessary player for completing the repair process. Without LIG4, fusion attempts ultimately collapse.
RAP1's Essential Binding Role
Further experiments highlighted that mutations preventing RAP1 from binding to DNA-PK compromised telomere protection, leading to chromosome fusion. However, restoring RAP1’s ability to obstruct LIG4 successfully reinstated protection.
Why This Matters: Implications for Aging and Cell Health
This discovery elucidates a longstanding enigma about how chromosomes maintain their integrity. As a fundamental molecular gatekeeper, RAP1 ensures a fail-safe mechanism, especially critical in aging or stressed cells. Given RAP1's conservation across species, it suggests an ancient defense mechanism that precedes Apollo's protective functions, creating dual layers of safeguard against chromosome fusion.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)