Surge in Tenecteplase Research for Stroke Treatment: A Game Changer?
2024-11-01
Author: Rajesh
Introduction
In recent years, tenecteplase has emerged as a competitive alternative to alteplase for the treatment of acute ischemic stroke. With its easy administration protocol, lower dosage, cost-effectiveness, and superior safety profile, tenecteplase is increasingly being considered in clinical settings. This article examines the growing body of academic literature surrounding tenecteplase—tracking its usage globally and analyzing trends from 1999 to 2023.
Methodology
Utilizing the Web of Science database, this study evaluated articles that included the keywords “Tenecteplase” and “Stroke.” The search yielded 576 journal articles, from which metadata on publication country, institution, keywords, and dates was meticulously analyzed.
Key Findings
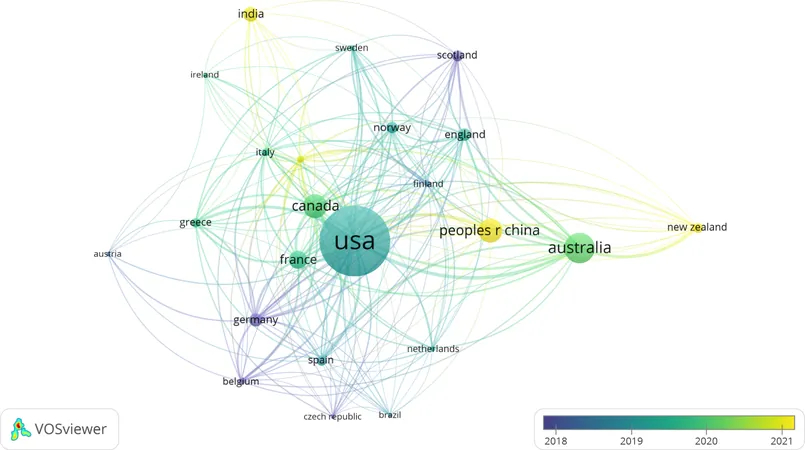
The United States led the pack with an impressive 39.93% of total publications (260 articles), followed by Australia (15.51%, 101 articles), with Canada and China sharing a close third (11.83%, 77 each). Among the most frequently mentioned keywords were tenecteplase (324 occurrences), alteplase (284), and thrombolysis (244). Notably, the University of Melbourne and the University of Calgary were the front-runners in research output.
In a remarkable year for tenecteplase research, 2023 saw the highest spike in publications, accounting for 24.3% of the total, suggesting a burgeoning interest in this treatment modality.
Conclusion
The escalating volume of published research on tenecteplase in 2023 holds promise for reshaping stroke treatment protocols. However, despite its demonstrated efficacy, tenecteplase remains an unapproved therapy by the U.S. FDA, as Genentech, its manufacturer, has yet to submit an application for formal approval. Given that strokes cause significant morbidity and mortality globally—ranked as the second leading cause of death—this gap in approval could impact thousands of lives.
The Global Burden of Stroke
Currently, strokes account for 143 million disability-adjusted life years worldwide, emphasizing the urgent need for effective treatments. The aging population has exacerbated the impact of strokes, particularly in low-income regions, where access to cutting-edge therapies remains limited.
Tenecteplase vs. Alteplase: An Economic Advantage?
Tenecteplase's growing popularity is partly due to a study demonstrating its cost-effectiveness. Over a decade, patients treated with tenecteplase incurred savings of €21 per individual, while also gaining more quality-adjusted life years compared to alteplase, which is currently the only FDA-approved thrombolytic for stroke. This economic edge, combined with favorable safety data, positions tenecteplase as a compelling alternative.
Recent Trends and Future Implications
The period from 2011 to 2021 saw an alarming 26.3% rise in stroke-related deaths in the U.S. alone. With tenecteplase showing such promise, the trend of increasing publications is a hopeful sign for what could become a critical shift in clinical practice. The time-saving benefits of tenecteplase—a mere five-second single bolus compared to alteplase's 60-minute infusion—enhance its practicality, especially in rural areas where timely treatment is crucial.
Collaborative Global Efforts
Collaboration across borders has resulted in significant advancements in research quality and quantity. While the U.S. leads in the number of publications, countries like India and New Zealand are recognizing the potential of tenecteplase, contributing to an enriched international dialogue on stroke treatments.
The Rise of Research Infrastructure
A fascinating aspect of this trend is the significant increase in funding directed toward stroke research, which surged to $443 million in 2023. This influx could catalyze further investigations into tenecteplase, potentially leading to its FDA approval and incorporation into treatment guidelines.
Limitations of Current Research
While the focus of this analysis lies in the Web of Science data, this approach has inherent limitations. Not all relevant studies may be covered, and researchers recommend integrating insights from multiple databases—such as PubMed and Scopus—to create a more comprehensive picture of tenecteplase’s trajectory in stroke research.
Conclusion
As the academic community rallies around tenecteplase, we may see a pivotal shift in how acute ischemic strokes are treated. With promising data backing its efficacy and an upward trajectory in research, tenecteplase could indeed revolutionize stroke care—if barriers to FDA approval are eventually lifted. The growing body of research is an indication that we are on the cusp of a new era in stroke treatment, and it’s time to keep a close watch on its developments!

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)