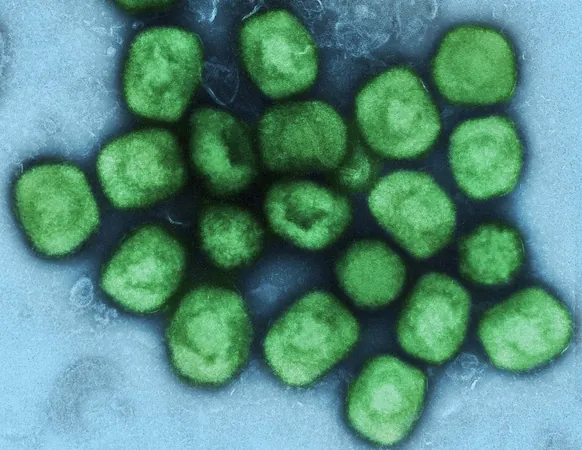
Single Dose of Imvanex Vaccine Offers 84% Protection Against Mpox, But Not for HIV Patients – What You Need to Know
2025-03-19
Author: Siti
Overview
Recent research from Universitätsmedizin Berlin has unveiled encouraging findings regarding the Imvanex vaccine, which was originally developed for smallpox. The study reveals that a single dose of this vaccine can provide up to 84% effectiveness against mpox; however, this does not extend to individuals living with HIV, who are advised solely to get a full two-dose regimen to achieve adequate protection. These groundbreaking results were published in The Lancet Infectious Diseases, underscoring the necessity for tailored health recommendations for at-risk groups.
A Landmark Study of Over 9,300 Participants
To thoroughly assess the vaccine’s protection levels, a research team headed by Prof. Leif Erik Sander conducted an extensive study involving over 9,300 participants, primarily men and transgender individuals who reported sexual interactions with men. The findings confirmed that the single-dose vaccine was notably effective in HIV-negative individuals, resulting in significantly fewer recorded mpox cases in the vaccinated group compared to their unvaccinated counterparts.
However, individuals living with HIV exhibited a minimal and statistically insignificant protective effect, leading researchers to advise these individuals to receive both doses of the vaccine as recommended. Prof. Sander emphasized that the immune response, particularly the production of T cells needed for effective protection after vaccination, is often compromised in people living with HIV.
Why Two Doses are Essential for Optimal Protection
Research indicates that a single dose may produce some level of immune response in healthy individuals, but for those with compromised immune systems such as individuals living with HIV, a second dose is critical for building adequate defenses against mpox. The study suggests that two doses can contribute significantly to longer-lasting immunity and may mitigate the severity of symptoms should a breakthrough infection occur.
Interestingly, vaccinated individuals who did contract the virus reported milder symptoms, such as fewer lesions and quicker healing processes. This points to the vaccine's potential role in reducing not just infection rates but also the severity of disease manifestations.
Safety Profile of the Vaccine
The tolerability of the mpox vaccine was assessed in a separate cohort of over 6,500 participants, revealing it to be generally safe and well-tolerated. The most common side effects were mild, primarily localized pain at the injection site, and less than 3% of individuals reported serious reactions such as fever or nausea.
Experts stress the importance of continued preventative measures, including safe sex practices and condom usage, to protect against other sexually transmitted infections, especially while immunity develops—typically within two weeks post-vaccination.
Looking Ahead: Future Research and Implications
The study initially focused on clade IIb of the mpox virus, but researchers express confidence that similar protective responses will be observed against the currently prevalent clade I, particularly in Africa, where outbreaks are surging.
Moving forward, long-term studies are planned to assess the durability of the vaccine's protective effects and the potential benefits of a third booster dose. With the emergence of mpox as a public health concern, these insights are pivotal in shaping effective vaccination strategies and safeguarding vulnerable populations against this virus.
Overall, as health authorities advocate for complete vaccination, it remains critical for all individuals, particularly those in at-risk categories, to be well-informed about their protective strategies against mpox and to adhere to vaccination guidelines for maximum health benefits.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)