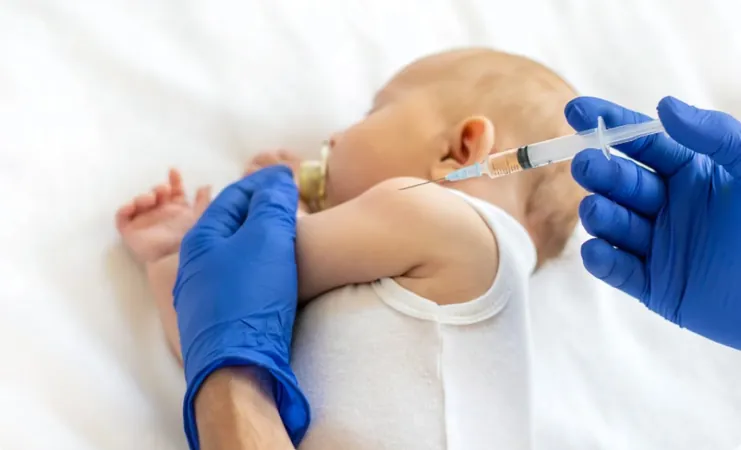
Singapore Takes a Bold Step: Introducing Beyfortus® to Shield Infants from RSV
2025-08-19
Author: Ming
Game-Changer in Infant Healthcare!
In a groundbreaking move, Singapore’s Health Sciences Authority (HSA) has officially approved BEYFORTUS (nirsevimab), the very first immunization geared specifically to protect infants against the potentially deadly respiratory syncytial virus (RSV). This vaccine is a vital safeguard for babies up to 24 months old, especially those entering their first RSV season.
Why RSV is a Serious Concern for Infants
Did you know that approximately 2 out of 3 infants contract RSV before their first birthday? This virus not only leads to harsh respiratory infections but is also a major reason for hospital visits in Singapore. Each year, around 1,804 children under 29 months end up hospitalized due to RSV-related complications such as bronchiolitis and pneumonia. In fact, many of these cases are in otherwise healthy, full-term babies.
Expert Consensus Calls for Action
In light of these alarming statistics, a group of pediatricians in Singapore has come together to emphasize the urgent need for RSV protection for all infants. Their expert consensus highlights the critical role of nirsevimab in alleviating the RSV burden on healthcare systems, urging authorities to consider its addition to Singapore’s National Immunisation Programme.
How BEYFORTUS Works: Protection at Its Best!
BEYFORTUS stands out as a long-acting antibody that can be administered as a single dose, providing newborns and infants with immediate protection against RSV. Remarkably, it does not require the activation of the immune system and can be delivered right at the onset of the RSV season.
Going Global with Safety!
The approval of BEYFORTUS is not confined to Singapore; this innovative vaccine has gained the green light in multiple key markets including the European Union, the United States, China, and Japan. Children around the globe are set to benefit from this significant advancement in pediatric health.

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)