Shocking New Insights on Tirofiban: What the FAERS Database Reveals About Real-World Risks!
2025-09-01
Author: Wei
Tirofiban's Role in Modern Medicine: A Life-Saving Antiplatelet Agent!
Antiplatelet medications are crucial in managing life-threatening conditions like heart attacks and strokes, with tirofiban standing out for its effectiveness. By binding competitively to platelet receptors, tirofiban prevents blood clots from forming, making it invaluable in treating acute coronary syndrome (ACS) and ischemic strokes.
Understanding the Risks: Tirofiban and Bleeding Complications
Despite its life-saving potential, tirofiban is notorious for a significant side effect: bleeding. Fortunately, its unique binding properties allow patients to regain normal platelet function fairly quickly after stopping the drug—usually within four hours—suggesting a relatively low risk in the longer term.
New Research Uncovers Real-World Data on Tirofiban's Safety Profile
To delve deeper into the safety of tirofiban, a comprehensive analysis was conducted using the FDA's adverse event reporting system (FAERS). This robust database collects voluntary reports from healthcare professionals and patients, shedding light on potential risks associated with tirofiban.
Key Findings: Bleeding and Beyond!
From a staggering 2,419 patient reports analyzed from 2004 to 2024, major reports highlighted bleeding as a prevalent issue, with hemorrhage and thrombocytopenia topping the lists of adverse reactions. Notably, reports included specifics like intracranial bleeding and gastrointestinal hemorrhages, hinting at the drug’s serious risks.
The Rising Use and Awareness: What's Behind the Surge?
The years 2019 to 2022 saw an uptick in reported adverse events, likely due to increased usage of tirofiban in clinical settings, especially for stroke therapy, and heightened awareness among physicians regarding the reporting system.
Crucial Insights from Clinical Trials: A Double-Edged Sword?
Recent trials underscore the delicate balance between the efficacy of tirofiban and its risks. While it holds promise for improving outcomes in ischemic stroke patients, concerns over its safety, particularly regarding intracranial hemorrhages, remain at the forefront of clinical discussions.
Uncovering New Adverse Events: What Surprised Researchers?
Additionally, new adverse reactions not documented in the drug's labeling surfaced, including instances of respiratory failure and coagulation disorders, amplifying the need for vigilant monitoring in patients treated with tirofiban.
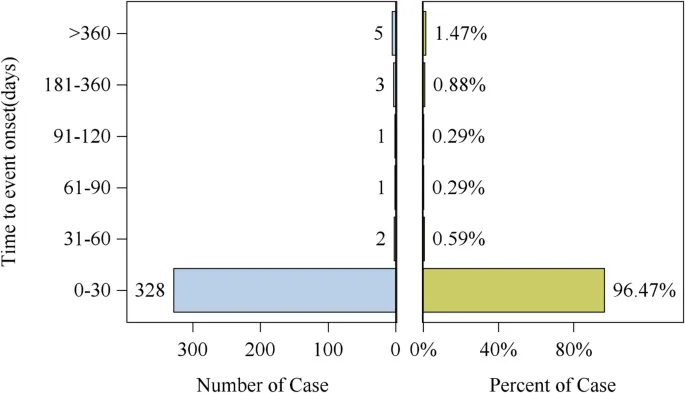
Future Directions: Monitoring and Managing Risks
Given the substantial findings, healthcare providers must ensure careful monitoring of patients starting tirofiban, particularly during the first month of treatment when most adverse events occur. This includes keeping an eye on hemoglobin levels to catch any signs of hidden bleeding early.
Conclusion: A Vital Choice in Cardiac Care Amidst Risks
While tirofiban remains a vital weapon in the fight against blood clots, awareness of its potential adverse effects is necessary for ensuring patient safety. The revelations from this study highlight the importance of ongoing research and surveillance in enhancing therapy while minimizing risks.

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)