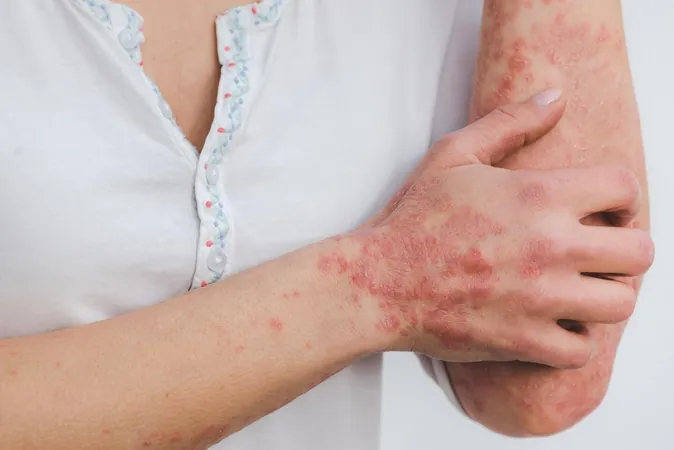
Shocking Link Revealed: Cancer Patients on Immune Checkpoint Inhibitors Face Higher Psoriasis Risks!
2024-11-08
Author: Nur
In a groundbreaking study from the National Defense Medical Center in Taiwan, researchers have unveiled alarming evidence that cancer patients undergoing treatment with immune checkpoint inhibitors (ICIs) are at a significantly heightened risk for developing psoriasis. This finding not only raises concerns for the patients affected but also has crucial implications for understanding other immune-related adverse effects associated with these therapies.
Immune checkpoint inhibitors have revolutionized cancer treatment over the past decade, harnessing the body’s immune system to target and destroy cancer cells. However, these potent therapies can come at a cost. They often lead to immune-related adverse effects due to the immune system mistakenly attacking healthy tissues, including skin, gastrointestinal organs, and even the lungs and endocrine system.
The study, titled "Psoriasis Risk With Immune Checkpoint Inhibitors," published in JAMA Dermatology, scrutinized data from Taiwan’s National Health Insurance database and the Taiwan Cancer Registry. Researchers focused on cancer patients at stage III and IV who were administered antineoplastic medications. The team’s objective was to examine the connection between ICIs and psoriasis, a chronic skin condition often characterized by painful red, scaly patches that can severely impact a patient's quality of life.
Out of a total of 135,230 patients (with an average age of nearly 63 years and a composition of 45% female), 3,188 were categorized as ICI users, while the remaining 132,042 were treated with traditional chemotherapy or targeted therapies, marking them as non-ICI users. The researchers monitored the incidence of psoriasis over the follow-up period to determine the increased risk.
Astoundingly, the analysis revealed that ICI users experienced a psoriasis incidence rate of 5.76 cases per 1,000 person-years, starkly contrasting with just 1.44 cases reported among non-ICI users. When adjusting for other risk factors, the likelihood of developing psoriasis for ICI patients surged—more than three times as likely (adjusted hazard ratio of 3.31) and over two times when factoring in additional risks (adjusted subdistribution HR of 2.43). These elevated risk estimates were consistent across various evaluative models and analyses.
The study underscored that ICIs disrupt the balance of the immune system, particularly agents targeting proteins like programmed cell death protein 1 (PD-1) and its ligand, PD-L1. This disruption causes an exacerbated immune response, leading T cells to erroneously attack healthy skin cells, which significantly raises the risk of psoriasis. The mechanism bears resemblance to how ICIs can incite other immune-related events by amplifying T helper cell inflammatory responses.
Notably, the loss of T-cell tolerance with ICIs could explain not just psoriasis but a spectrum of immune-related adverse events that can range from mild to life-threatening, impacting various organs within the body.
This revelation adds a vital layer of comprehension to the multifaceted ways ICIs can lead to psoriasis and other immune-related complications. Previous studies have indicated that elevated levels of programmed cell death can exacerbate the severity of psoriasis, emphasizing the importance for healthcare professionals to remain vigilant regarding these potential adverse effects.
As cancer treatment continues to evolve, it becomes increasingly critical for clinicians to balance the therapeutic benefits of immune checkpoint inhibitors against the risks they carry, ensuring the highest standard of care for patients battling cancer.




 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)