Shocking Discovery: Pregnant Mice Experience Permanent Gut Transformations!
2025-03-19
Author: Arjun
A Groundbreaking Study
In a groundbreaking study conducted by researchers at the Francis Crick Institute, it has been revealed that pregnancy causes significant and potentially permanent changes to the small intestine in mice. These findings open up new avenues for understanding how reproduction impacts organ physiology and nutrient absorption in mammals.
Physiological Changes During Reproduction
Traditionally, many female animals undergo physiological changes during reproduction, yet the gut's response to pregnancy has only begun to capture the scientific community's interest. Past research uncovered that even fruit flies experience gut expansion linked to reproductive stages, but mice present a unique case for investigation.
Study Observations
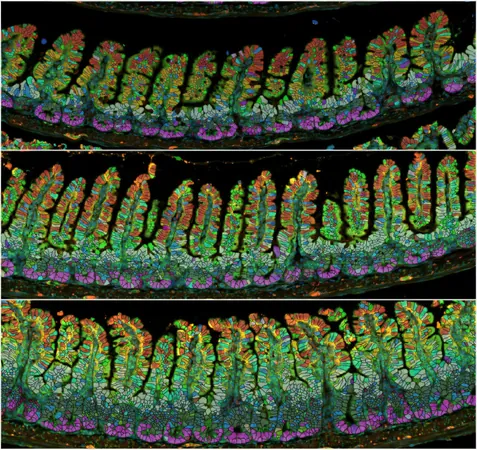
According to the study published in the journal *Cell*, researchers observed that the small intestine in pregnant mice began to grow just a week into gestation, ultimately becoming an astonishing 18% longer by day 18 of pregnancy. What’s even more intriguing is that this elongation persists after birth, remaining unchanged for up to 35 days post-lactation, suggesting that these adaptations are not merely temporary adjustments but rather a fundamental alteration of the gut structure.
Impact of Second Pregnancy
Notably, the restructuring becomes further pronounced after a second pregnancy, indicating that these changes could better equip mothers to nourish their offspring through improved nutrient absorption. The team noted that within the small intestine, villi – the tiny finger-like projections responsible for nutrient absorption – and crypts – the sites for new cell generation – transformed both in size and depth during this process. However, after weaning, both reverted to their original pre-pregnancy dimensions within a week, revealing a delicate balance between adaptation and regulation.
Dietary Manipulations and Findings
To dig deeper, the researchers manipulated the diets of the pregnant mice, including the incorporation of probiotics, only to find that these significant physiological changes occurred independent of nutritional variations. The results underscored a robust interplay of hormones and genetic expressions activated during pregnancy, primarily affecting the enterocytes, the cells responsible for absorbing nutrients.
Role of SGLT3a Protein
One fascinating aspect of the findings revolves around a specific membrane protein, SGLT3a, which showed heightened activity during early pregnancy. This protein is distinct because it reacts not to glucose, but to sodium and protons, driving nearly half of the villi growth associated with reproductive changes. Excitingly, when sodium was supplemented in the diets of virgin females, similar villi growth was observed, indicating that hormonal shifts triggered by reproduction likely play a role in activating genes linked to SGLT3a.
Expert Insights
Irene Miguel-Aliaga, leading the Organ Development and Physiology Laboratory at the Crick, emphasized the remarkable adaptability of the gut in response to pregnancy, likening these changes to an energy trade-off strategy. The enduring longer gut could prime the body for subsequent pregnancies while temporarily shortening villi might limit unnecessary nutrient absorption when it's not required.
Future Research Directions
Tomotsune Ameku, a co-author of the study, expressed optimism about understanding how pregnancy alters the physiology of other mammals, including humans. He stressed that while pregnancy in mice offers crucial insights, the implications for human health remain an open question, especially as modern lifestyles differ markedly from the past in terms of diet and reproductive patterns.
Conclusion and Next Steps
The team at the Francis Crick Institute is now setting their sights on further studies investigating whether similar remodeling occurs in other cell types within the mouse intestine during pregnancy — and more importantly, whether these phenomena are present in humans. As this research unfolds, we may unravel more mysteries surrounding pregnancy's profound impact on bodily functions, which could pave the way for innovative approaches to maternal health.
Stay tuned as we delve deeper into this fascinating area of study and how it can influence our understanding of human biology and health!

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)