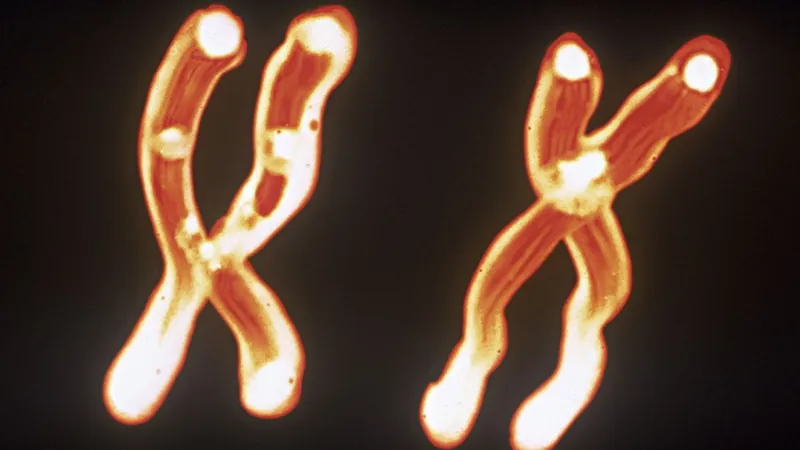
Shocking Discovery: Mom's X Chromosome May Speed Up Brain Aging!
2025-01-22
Author: Daniel
Introduction
A groundbreaking study has revealed that the X chromosome inherited from mothers could potentially accelerate brain aging, sparking debates about gender differences in cognitive decline. Conducted on mice, this intriguing research opens doors to understanding how these findings may apply to humans, raising questions about tailored therapeutic approaches for brain health.
Study Background
Dr. Dena Dubal, a professor of neurology at the University of California, San Francisco, led the investigation into the aging mechanisms of male and female brains. Her study highlights a fascinating observation: while females generally show resilience to various aging markers, including a longer lifespan and reduced rates of certain dementias, they paradoxically suffer from Alzheimer’s at greater rates. Despite this, studies indicate that women with Alzheimer's often live longer than their male counterparts.
Research Methodology
The research team, including postdoctoral fellow Samira Abdulai-Saiku, focused on the differences between X chromosomes inherited from mothers and fathers. Mice, which possess either a pair of X chromosomes (females) or one X and one Y (males), were examined to elucidate how these sex chromosomes impact cognitive health. Remarkably, females inherit two X chromosomes, but one is usually silenced, allowing diverse genetic expression that may contribute to resilience against cognitive decline.
Experimental Findings
In a unique experimental setup, researchers silenced the paternal X chromosome in female mice, isolating the influence of the maternal X chromosome. They unveiled that while young "Mom-X" mice performed similarly to their peers in cognitive tests, older mice exhibited pronounced cognitive decline, particularly in spatial and working memory tasks.
Biological Mechanisms
To understand the biological mechanisms behind these age-related changes, the team examined the hippocampus—the brain's critical memory hub—analyzing DNA for epigenetic markers that indicate biological aging. Their findings revealed that the Mom-X mice displayed more severe aging signs in the hippocampus relative to those with both X chromosomes active.
Gene Identification and Implications
Furthermore, they pinpointed three key genes—Sash3, Tlr7, and Cysltr1—that were inactive on the maternal X chromosome but highly active on the paternal X. Remarkably, by utilizing CRISPR gene-editing technology to reactivate these genes in older mice, researchers noted significant improvements in spatial learning and memory.
Potential Human Relevance
These revelations have profound implications for humans, especially since the identified genes are linked to immune protection, although their functions in brain neurons remain under investigation. Dubal and her team are eager to explore how these findings impact males, who inherit their only X chromosome from their mothers. Could this lead to greater brain aging in men? Dubal speculates that the effects might indeed be influenced by the activity of maternal X genes.
Conclusion and Future Directions
Importantly, these results, derived solely from rodent models, necessitate further exploration within human brain tissues to validate their relevance. This unique and pioneering research could reshape our understanding of dementia, disentangling it from other sociocultural factors like education—potentially guiding more personalized treatment plans for patients.
Buckley urges the scientific community to shift away from a "one size fits all" approach to treating cognitive decline. With this bold new research paving the way, the future may offer targeted therapies that take into account the distinct biological drivers of brain aging. Stay tuned, as we uncover more about this critical area of study that could redefine how we approach cognitive health across genders!

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)