Scientists Uncover Shockingly Fast Reaction Between Criegee Intermediates and Water!
2025-04-28
Author: Siti
What Are Criegee Intermediates?
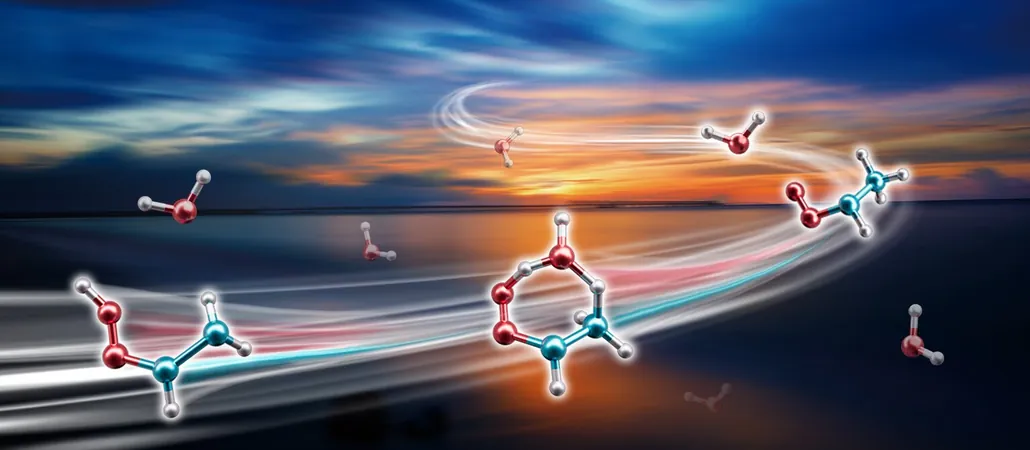
Criegee intermediates (CIs) are incredibly reactive molecules created when ozone interacts with alkenes in our atmosphere. These intermediates are essential for producing hydroxyl radicals—nature’s own "cleaning agents"—and aerosols that can dramatically affect both climate and air quality.
A Game-Changer in Atmospheric Chemistry!
Among the various CIs, syn-CH3CHOO stands out, comprising a whopping 25% to 79% of all CIs during different seasons. Scientists previously thought that syn-CH3CHOO predominantly dissipated through its own decomposition. However, groundbreaking research led by a team from the Dalian Institute of Chemical Physics (DICP) has shattered this old belief.
The Jaw-Dropping Discovery!
In a revelatory study published in *Nature Chemistry*, researchers discovered that syn-CH3CHOO reacts with atmospheric water vapor roughly 100 times faster than earlier theories suggested. Using cutting-edge laser technology, they were able to measure this rapid reaction, leading to a striking new understanding of atmospheric chemistry.
What Drives This Acceleration?
To delve into the mechanism behind this surprising speed, the team created an intricate 27-dimensional model to explore the potential energy interactions at play. They identified a unique "roaming mechanism," highlighting the significant dipole-dipole forces between molecules. This interaction allows the molecules to remain in proximity, drastically increasing the likelihood of a reaction.
Implications for Climate Science!
The findings challenge the long-held idea that unimolecular decomposition is the primary route for syn-CH3CHOO removal. This revised understanding could provide scientists with improved models for predicting climate change and air quality. The study emphasizes the necessity of integrating precise experimental data with advanced simulations to accurately forecast the complexities of chemical reactions in Earth's atmosphere.

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)