Revolutionizing Stroke Trials: How Electronic Consent is Breaking Down Barriers
2025-06-02
Author: Sarah
Unlocking New Avenues in Stroke Clinical Trials
A groundbreaking analysis of a major randomized clinical trial has unveiled the transformative potential of electronic informed consent (eConsent) in acute ischemic stroke studies. Not only does eConsent promise to boost enrollment rates, but it also enhances the adherence to consent documentation, paving the way for more inclusive stroke research.
Insights from Leading Researchers
Recently published in the esteemed journal *Stroke*, this study is spearheaded by Iris Davis from the University of Cincinnati and Christopher Streib from the University of Minnesota, along with their research team. They reveal that eConsent significantly increases individual site enrollment and facilitates remote consent, making the informed consent process more efficient compared to traditional paper methods.
The Struggle with Traditional Methods
Securing timely informed consent has long been a "key barrier" in stroke clinical trial recruitment. While eConsent offers a modern solution, its adoption in these urgent trials has been limited and often understudied. The researchers aimed to bridge this gap by analyzing eConsent's role in the MOST trial, a Phase 3 study assessing two blood-thinning medications in adult ischemic stroke patients.
Data That Speaks Volumes
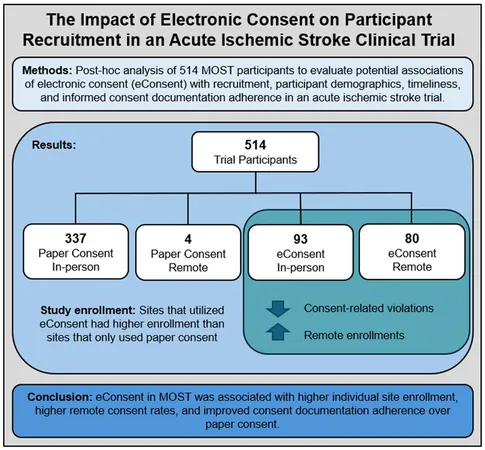
Findings from the MOST trial, presented at last year's International Stroke Conference and detailed in the *New England Journal of Medicine*, reflected no significant 90-day clinical benefits from the medications tested. However, Davis and her team discovered compelling statistics regarding consent methodologies. Out of 514 participants, eConsent was utilized by 173 individuals—showing a marked preference for electronic over traditional methods.
A Shift Towards Remote Accessibility
The analysis pointed out that eConsent was completed remotely more frequently than paper consent, fundamentally changing the game for patient enrollment. Remarkably, less than 1% of participants were consented remotely using paper, highlighting the greater practicality of eConsent for remote patients, especially in urgent acute care scenarios.
Enhancing Compliance and Reducing Errors
The research demonstrated that the adherence to consent documentation was significantly better among participants who used eConsent compared to those using paper forms. This translated to fewer data clarification requests and a decrease in reportable unanticipated events, underscoring the efficacy of electronic management in clinical trials.
An Eye Towards the Future
Experts like Streib advocate for the broad application of eConsent in various stroke trials, including those exploring thrombectomy and novel treatment protocols. They acknowledge that while challenges remain—like regulatory hurdles and the need for infrastructure to support eConsent—its advantages could be game-changing, particularly for patients in rural areas who often face barriers to participation.
In Conclusion: A Pivotal Change in Clinical Trials
As health care continues to evolve, electronic methods are replacing outdated paper documentation, offering a more seamless way to manage medical information. eConsent stands out as a promising solution to enhance patient participation in clinical trials, making it easier for researchers and healthcare providers to navigate the complexities of informed consent.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)