Revolutionizing Plastic Waste: How Electrochemistry is Transforming Polymer Upcycling
2025-09-19
Author: Arjun
Turning Trash into Treasure: The Future of Polymer Recycling
High-performance materials like carbon and plastic composites are game-changers in reducing fuel consumption and emissions in vehicles, airplanes, and electric cars. However, the very properties that give these materials their strength and lightness pose a significant challenge: they are notoriously difficult to recycle.
To combat the growing concern of landfill disposal and inefficient recycling methods, researchers at the University of Illinois Urbana-Champaign have embarked on an exciting journey to develop a new way to manufacture carbon-fiber reinforced plastics that can be easily deconstructed at the end of their life cycle.
Innovative Energy Solutions: A New Approach to Waste
In a breakthrough study, chemistry professor Jeffrey S. Moore and his team have discovered a method that employs electricity to reshape recycling byproducts into materials of value. This process is outlined in their recent paper published in 'Nature Synthesis,' titled "Dual C–H Functionalization of Polyolefins for Covalent Adaptable Network Formation via Cooperative Electrolysis." Moore emphasizes that their work closes a vital loop in the lifecycle of carbon fiber composites.
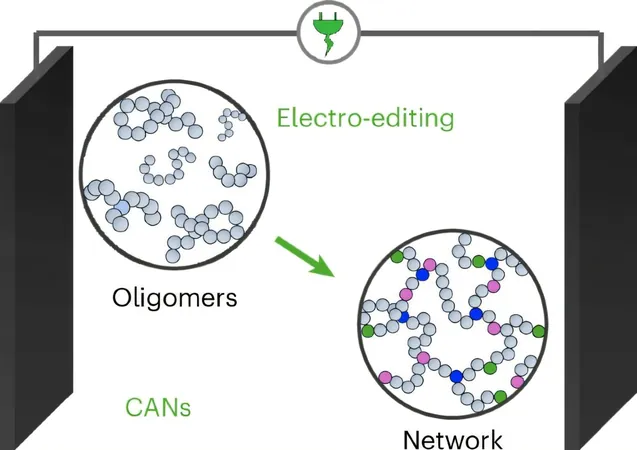
By utilizing electrolysis—an electric current-driven chemical reaction—the researchers have found a way to modify the molecular structure of waste fragments, known as oligomers, left over after carbon fiber extraction.
From Waste to Wealth: The Science Behind the Procedure
The fragments derived from the carbon-fiber reinforced polymer composite (CFRP) consist of oligomers, which are short chains of repeating molecules. While breaking down CFRPs recovers valuable carbon fibers, it results in waste oligomers that lack significant mechanical properties.
However, through innovative electrolysis, the research team modifies the oligomer structure at two distinct locations, effectively turning waste into a new network formation. This process involves adding functional groups to the oligomer chain, allowing for the reassembly of these fragments into robust structures known as Covalently Adaptable Networks (CANS). This breakthrough means that these materials not only regain their strength but can also be repurposed for new applications.
Broad Applications and Environmental Impact
The potential applications for these rejuvenated thermoset materials are vast—ranging from automotive components to electronic devices and protective coatings. Moore states, "By creating a sustainable pathway for reusing oligomers that would otherwise be discarded, this work contributes to net-zero waste manufacturing and highlights the potential of electrochemistry for polymer upcycling."
A Step Towards Sustainable Manufacturing: The Research Implications
This study marks a significant advancement in the field, showcasing the first scalable method for dual carbon–hydrogen functionalization along complex polymer backbones. Previous methods had been limited to minor adjustments or end-group chemistry, failing to directly transform the oligomer chain at critical sites.
The team's findings reveal that certain carbon-hydrogen sites in branched oligomers are more reactive than previously thought, paving the way for new chemical innovations.
With plans to extend this revolutionary electrochemical method to other materials—such as polybutadiene, an essential player in synthetic rubber—the research team is poised to redefine the future of sustainable material reprocessing.

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)