Revolutionizing Photocatalysis: Key Breakthrough Unlocks New Levels of Efficiency
2025-05-12
Author: Siti
Photocatalysis—the stunning process where light drives chemical reactions through a catalyst—is set to redefine our approach to sustainable technologies. It holds the promise for innovations in hydrogen production via water splitting, carbon dioxide reduction, and environmental purification, using just the power of sunlight. As cities and communities strive for sustainability, the spotlight is on photocatalysis as an essential tool for the future.
Yet, with its complex series of steps—light absorption, carrier excitation, transport, and surface reactions—it’s been a daunting task to identify which part of the process is slowing things down. This has hindered efforts to optimize photocatalytic reactions for enhanced efficiency.
In an exciting new study, researchers from the Graduate School of Advanced Science and Technology at the Japan Advanced Institute of Science and Technology have made a groundbreaking leap forward. Led by Research Assistant Professor Yohei Cho and Professor Toshiaki Taniike, they have devised a unique methodology that pinpoints bottleneck metrics, shedding light on the rate-limiting steps within photocatalysis. Their findings, recently published in the Journal of Materials Chemistry A, could change the game.
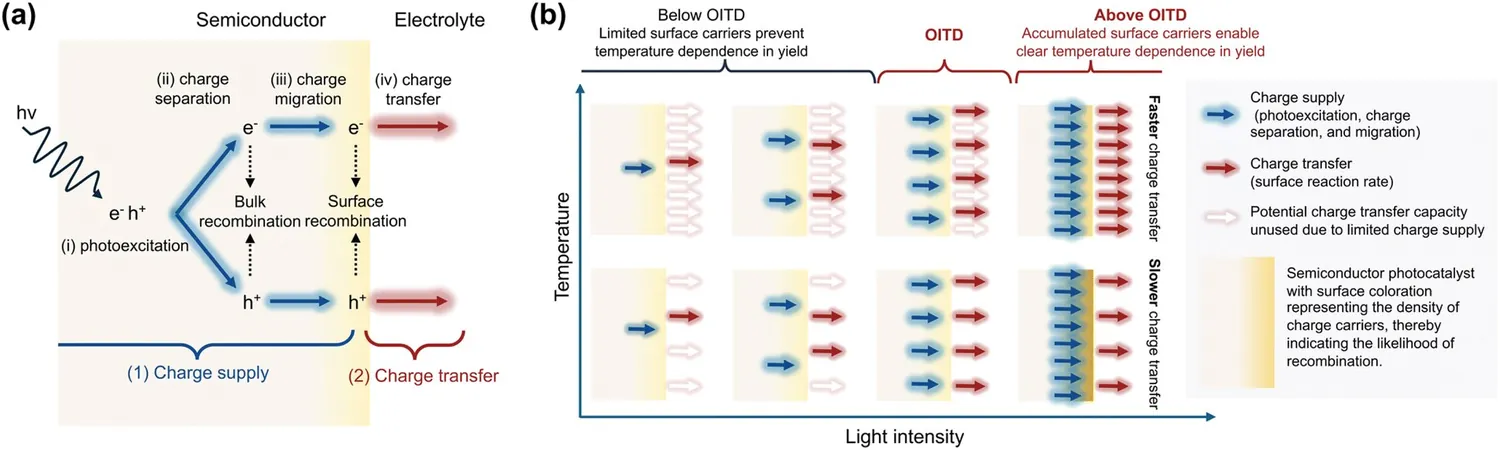
Dr. Cho elaborates, "We divided photocatalytic reactions into two main areas: charge supply and charge transfer. The former refers to the delivery of excited carriers to the surface, while the latter deals with redox reactions. We introduced the Onset Intensity for Temperature Dependence (OITD)—a key metric that shows the temperature at which reaction rates begin to change, allowing us to differentiate between these two critical processes."
The team examined photocatalytic reaction rates across different temperatures and light intensities to identify the OITD. By using methylene blue decomposition as a model reaction, they evaluated two common photocatalysts: titanium dioxide (TiO2) and zinc oxide (ZnO).
The results were enlightening. TiO2 demonstrated sensitivity to temperature only at high light intensities, indicating that it is primarily constrained by charge supply. Conversely, ZnO showed temperature sensitivity even at lower light intensities, suggesting its performance is more limited by surface reactions. This distinction reveals that different materials exhibit unique rate-limiting behaviors.
The researchers further discovered that improving surface accessibility through nanoparticle formation is more crucial for enhancing charge supply than merely increasing crystallinity. This paves the way for innovative strategies in photocatalytic material design.
Dr. Cho highlights the importance of their research: "This diagnostic method allows for the rational design of photocatalysts aimed at solar-driven hydrogen production, carbon dioxide reduction, and environmental remediation. It enables swift material screening and targeted optimization tactics—like co-catalyst loading or nanostructuring—to develop practical solar energy applications."
Ultimately, this breakthrough could expedite the advancement of sustainable energy and purification technologies, significantly contributing to carbon neutrality and cleaner ecosystems.
In summary, the introduction of OITD as a diagnostic tool offers a clear and powerful means of identifying whether photocatalytic reactions are limited by charge supply or surface charge transfer. This revolutionary approach sets the stage for smarter catalyst designs and significantly improved reaction efficiencies.



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)