Revolutionizing Peptide Drug Discovery: The Power of a Natural Enzyme
2025-08-25
Author: Wei
Enticing Breakthrough in Medicine
A groundbreaking discovery from a collaborative team at the University of Utah and Sethera Therapeutics is set to reshape the future of peptide drug development, paving the way for treatments against diseases once deemed "undruggable." Published in the prestigious Proceedings of the National Academy of Sciences, this cutting-edge research unveils a game-changing natural enzyme, PapB.
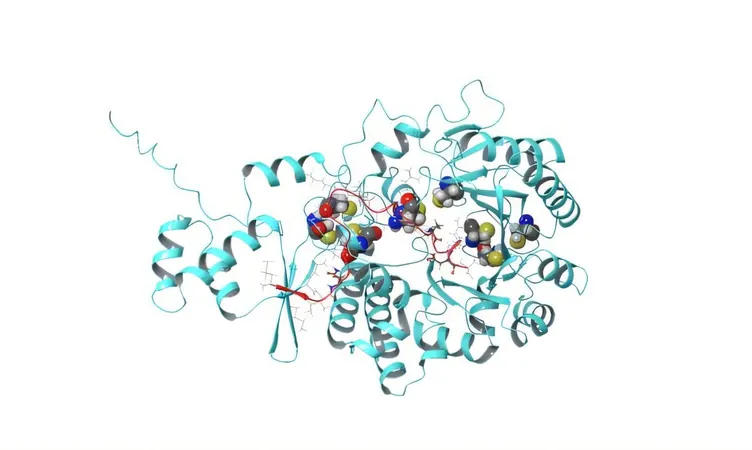
The Secret Weapon: PapB
PapB employs a unique mechanism to transform linear peptides into stable, circular forms known as macrocycles. What distinguishes PapB is its remarkable blend of flexibility and precision—enabling it to work with a diverse array of building blocks, including those traditionally rejected by biology, forging a single, reliable bond. This process simplifies the journey from linear to robust ring-shaped molecules, enhancing stability and resistance to breakdown essential for effective drug development.
Streamlining Drug Development
Conventionally, peptide drugs rely on disulfide bonds, which are vulnerable to decomposition in the body, or elaborate chemical methods that are costly and time-consuming. However, PapB streamlines this entire process, facilitating the creation of durable, "stapled" peptides that can be engineered with exceptional ease. This innovation opens vast new avenues for peptide medicines, notably improving cell penetration and oral dosing capabilities—two critical features for advancing therapeutic efficacy.
A New Era of Therapeutics
According to Karsten Eastman, CEO and Co-founder of Sethera Therapeutics, the breakthrough allows peptides to behave like both small molecules and biologics simultaneously. "This enzyme empowers us to generate a lasting thioether 'staple' across a broad spectrum of backbones in one enzymatic step, drastically enhancing our capacity to target difficult biological challenges," he emphasized.
Unlocking New Potential
This pioneering work positions PapB as a game-changing thioether ligase, opening up unprecedented opportunities for peptide drug discovery. By merging biological selectivity with chemical versatility, the researchers aim to create next-gen peptide therapeutics that address hard-to-target ailments.
The Impact of PapB
Dr. Vahe Bandarian from the University of Utah highlights the remarkable capabilities of PapB, stating, "It's not just about promiscuity; it’s about controlled promiscuity. PapB can accommodate a range of amino acids and precisely place a thioether where needed, enabling access to stable macrocyclic peptide scaffolds that were previously inaccessible through synthetic methods alone."
Conclusion: A Tool for the Future
This discovery arms researchers with a powerful one-step approach for generating stable, diverse peptide macrocycles with drug-like properties. The implications are immense for biotech and pharmaceutical teams eagerly seeking next-generation therapies in realms where conventional drugs have fallen short.



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)