Revolutionizing Green Chemistry: A Sustainable Approach to Epoxidizing Corn Oil
2025-09-01
Author: Wei Ling
Unlocking the Potential of Sustainable Materials
In a world increasingly aware of environmental challenges like fossil fuel depletion and air pollution, the need for sustainable materials has never been more pressing. Traditional petrochemical resins, lauded for their strength and durability, fall short when it comes to biodegradability and safety. As such, the quest for bio-based products has surged, aiming to utilize renewable sources for greener chemical processes.
Corn Oil as a Game-Changer
Corn oil, rich in linoleic acid, stands out as a prime candidate for sustainable chemical processes. Its high level of unsaturation offers multiple double bonds ready for epoxidation, making it superior to oils like palm or coconut oil, which contain more saturated fats. With an abundance of corn oil available at relatively low costs, it could play a crucial role in the next wave of sustainable chemical manufacturing.
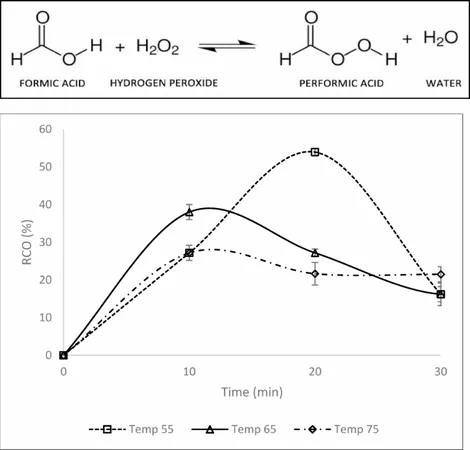
The Power of Epoxidation
Epoxidation, a key chemical reaction, involves adding oxygen to carbon-carbon double bonds, resulting in the formation of epoxides. Utilizing an in situ performic acid method allows safe and effective epoxidation of unsaturated fatty acids like those found in corn oil. This innovative process generates performic acid in the reaction itself, sidestepping the need for hazardous pre-synthesized peracids.
Catalytic Advancements for Efficiency
This research explores a catalytic process for epoxidizing linoleic acid-rich corn oil. By employing a tailored catalyst and optimizing the reaction conditions, the study aims to enhance efficiency and scalability—paving the way for greener chemical manufacturing. The focus is on balancing temperature, catalyst loading, and the hydrogen peroxide ratio to maximize oxirane yield while minimizing environmental impacts.
Key Findings on Epoxidation Dynamics
Experiments revealed that adjusting the temperature is crucial for achieving optimal relative conversion to oxirane. At specific temperature thresholds, the reaction's productivity peaked before declining, underscoring the sensitivity of the process. Similarly, varying the molar ratio of hydrogen peroxide highlighted the delicate balance required for maximizing yields—excessive hydrogen peroxide resulted in instability for the oxirane ring.
Sulfation and the Sweet Spot
Utilizing sulfuric acid as a catalyst proved effective, with an optimal loading found at 3 grams, which significantly enhanced the reaction. Too much catalyst, however, can trigger unwanted side reactions. These findings paint a clearer picture of sulfuric acid’s role and provide invaluable insights for future work in embracing corn oil as a viable raw material.
From Theory to Practice: Kinetic Modeling
By applying kinetic modeling, the researchers developed equations that depict the epoxidation process and the vital pathways involved. These models lay the groundwork for predicting outcomes and improving the epoxidation process through data-driven insights.
Conclusion: A Sustainable Future for Epoxidized Corn Oil
With results indicating that corn oil could serve as a sustainable alternative to conventional petroleum-based epoxies, this breakthrough could reshape the chemical landscape. The study not only enhances our understanding of epoxidation kinetics but also advocates for investing in bio-based resources—bolstering waste reduction and paving the way for a greener future in materials science.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)