Revolutionizing Gas Dynamics: The Maxwell-Boltzmann Distribution Gets a Modern Makeover
2025-07-15
Author: Jia
The Legacy of Maxwell and Boltzmann
For over 150 years, the Maxwell–Boltzmann distribution has defined our understanding of molecular speeds in ideal gases, thanks to the groundbreaking work of James Clerk Maxwell and Ludwig Boltzmann. This foundational concept remains a staple in physical chemistry and statistical mechanics classes for aspiring scientists.
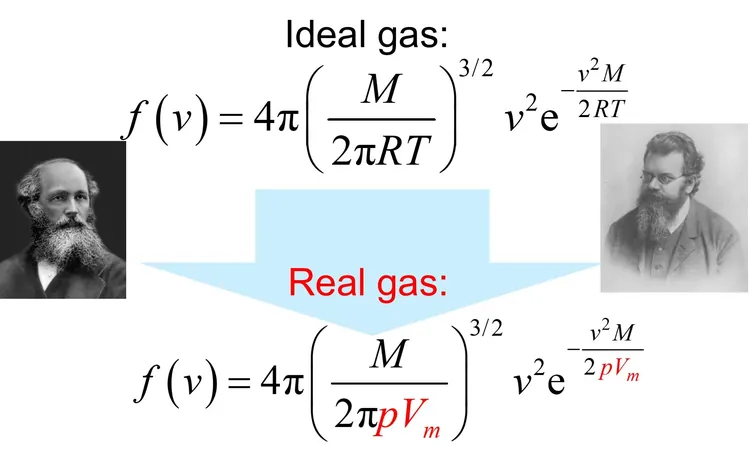
A Breakthrough in Real Gas Theory
But what about real gases? A recent theoretical paper has introduced a game-changing formula that extends the Maxwell–Boltzmann distribution to these non-ideal gases, which often fail to conform to the ideal gas law due to significant molecular interactions.
Unveiling the New Formula
This innovative approach leverages probability theory to formulate a generalized distribution applicable to any gas sample, ideal or otherwise. The core insight? The physical properties of gases do not depend on direction. This observation leads to a robust mathematical proof and highlights the essential characteristics of molecular velocity.
Implications for Molecular Behavior
The new derivation relies on a key principle: the velocity components of a molecule must adhere to a normal distribution, reflecting the widely accepted central limit theorem. The outcome? A comprehensive three-dimensional speed distribution modeled after a chi distribution with three degrees of freedom.
Transforming Physical Properties
What sets this new formula apart is its ability to connect to practical thermodynamic parameters. Instead of relying solely on temperature, it incorporates the product of pressure and molar volume, allowing scientists to directly calculate average molecular speeds, collision rates, and even diffusion coefficients—tasks that were previously complex or elusive.
A Call for Further Exploration
This development not only refines our calculations for real gases but also opens the door for standard probability theory to enhance our comprehension of various non-ideal systems. The future of gas dynamics may be brighter and more precise than ever, reshaping the landscape of thermodynamics.
Meet the Mind Behind the Breakthrough
Dr. Gábor Lente, a prominent figure in the field, holds a PhD from the University of Debrecen and is a full professor of chemistry at the University of Pécs. His expertise lies in mathematical reaction kinetics, and he is dedicated to empowering the next generation of scientists through his teachings.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)