Revolutionizing Drug Production: A Breakthrough in Biomolecule Synthesis without External Catalysts
2025-06-16
Author: Wei
A Game-Changer in Pharmaceuticals
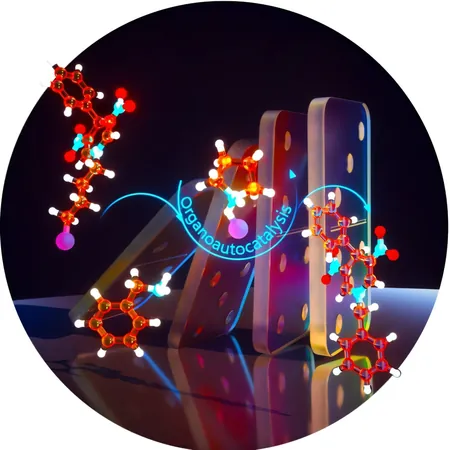
Imagine a future where bioactive molecules and pharmaceuticals can be produced without relying on harmful enzymes or toxic metals. Thanks to groundbreaking research from chemists at Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), this vision may soon become a reality. Their innovative method utilizes an in situ-formed organoautocatalyst, enabling highly efficient chemical synthesis of bioactive cyclic amine compounds under mild conditions.
Cyclic Amines: A Key Player in Medicine
Cyclic amines, ring-shaped molecules made from nitrogen and carbon, are increasingly vital in the fields of medicine and biochemistry. Among these, dihydropyridine stands out—a six-membered ring comprising five carbon atoms and one nitrogen atom. Dihydropyridine compounds are instrumental in lowering blood pressure, and their tunable fluorescence makes them potential candidates for cutting-edge photoelectronic materials.
Tailoring Molecule Properties with Precision
"The nitrogen atom in dihydropyridines can bind with various molecules, offering targeted control over their biological properties," explains Prof. Dr. Svetlana Tsogoeva, leading the research team at FAU's Department of Chemistry and Pharmacy. This adaptability in chemical structure could lead to more effective medicinal applications.
The Challenges of Traditional Synthesis
Historically, synthesizing these important compounds has been fraught with difficulties—often requiring complex enzyme catalysts or costly and hazardous metals, along with extreme reaction conditions. This not only inflates production costs but also results in toxic waste, posing additional risks, especially in pharmaceutical production.
A Breakthrough Method: No External Catalysts Needed
The Tsogoeva team's new approach completely eliminates the need for external catalysts while maintaining high effectiveness. By utilizing pyrrolidinium salt formed during the synthesis process, they have created an organoautocatalyst that accelerates reactions to yield an impressive 95% efficiency—all at room temperature.
Opening New Frontiers in Chemical Synthesis
"This innovative method goes beyond simply imitating nature—it paves the way for novel chemistry in carbon-nitrogen compounds," asserts Tsogoeva. With such advancements, the future of sustainable pharmacological production looks brighter than ever.

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)