Revolutionizing CO2 Reduction: The Power of Hydrogen Bonding!
2025-04-21
Author: Li
Transforming Waste CO2 into Energy and Profit
The world is facing an environmental crisis, but there's an unprecedented opportunity on the horizon! The catalytic conversion of waste CO2 into valuable fuels and chemicals not only promises to protect the environment but also boost economic development. Among the most exciting approaches is the electrocatalytic CO2 reduction reaction (CO2RR), which efficiently turns CO2 into clean chemical energy under mild conditions.
Conquering Energy Barriers with Innovative Engineering
However, there's a catch—the formation of the *COOH intermediate often presents a high energy barrier, hindering the overall efficiency of the CO2RR process. Enter a groundbreaking approach from researchers at the University of Science and Technology of China, led by Professors Jiang Hai-Long and Jiao Long, who have taken inspiration from enzyme catalysis to develop an innovative strategy!
A Game-Changer: Hydrogen-Bonding Microenvironment
These forward-thinking scientists engineered a hydrogen-bonding microenvironment around the catalytic sites, stabilizing the troublesome *COOH intermediate. Their pioneering research has just been published in the Proceedings of the National Academy of Sciences, highlighting their revolutionary method.
Key Innovations in Catalytic Materials
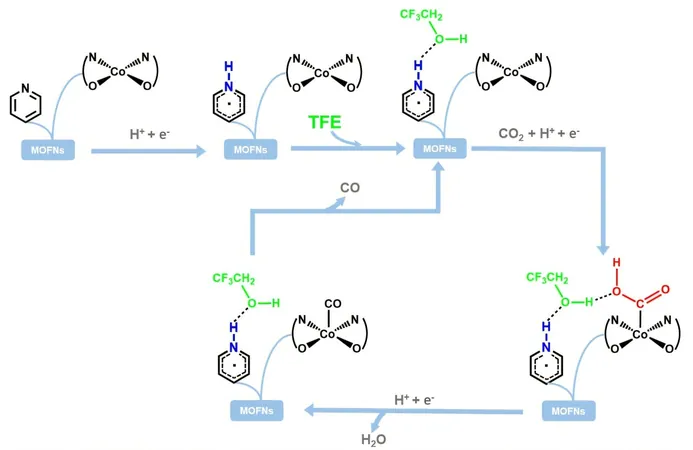
The team skillfully co-grafted catalytically active Co(salen) units with pyridyl-substituted alkyl carboxylic acids onto Hf-based MOF nanosheets, resulting in a novel material dubbed Co&X-PyCn/MOFNs. This clever combination allows for precise control over the positioning of nitrogen atoms in pyridine, enabling a tailor-made microenvironment at the atomic level.
Unmatched Performance of Enhanced Catalysts
Of all the catalysts tested, the Co&p-PyC3/MOFNs showcased astonishing improvements in catalytic activity and selectivity for electrochemical CO2 reduction, outshining traditional Co/MOFNs and other variants. This breakthrough not only sets a new standard but also emphasizes the true potential of smart catalyst design.
Unveiling the Mechanism Behind Success
But wait, there’s more! The researchers discovered that during the CO2 reduction process, pyridine is converted into a reactive pyridinyl radical (PyrH•). This species, combined with trifluoroethanol in the electrolyte, forms a unique triad intermediate that stabilizes the *COOH intermediate, dramatically reducing the energy barrier for the reaction. This revelation sheds light on how microenvironment engineering can optimize catalysis.
A Beacon of Hope for Future Research
This research unequivocally demonstrates the critical role of microenvironment modulation in enhancing catalytic processes. It not only paves the way for future studies into more efficient catalysis but also provides hope for sustainable energy solutions. The journey towards a cleaner and greener future is gaining momentum—one hydrogen bond at a time!



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)