Revolutionizing Chemistry: Australian Researchers Unleash Quantum Computing to Mirror Molecules in Action!
2025-05-17
Author: Daniel
Imagine a molecular dance where light triggers a breathtaking flurry of quantum transformations! Electrons leap between energy levels, atoms vibrate with energy, and chemical bonds twist and turn—all within mere millionths of a billionth of a second. This intricate ballet is the heartbeat of nature, influencing everything from how plants harness sunlight during photosynthesis to how sunlight can damage DNA, not to mention its pivotal roles in solar technology and innovative cancer treatments.
Despite their crucial role, accurately simulating these light-driven chemical processes has long been a Herculean task. Traditional computers falter under the immense computational demands of modeling such quantum behavior.
Enter quantum computers—devices that operate on the very principles of quantum mechanics, making them perfectly suited for tackling complex chemical simulations.
A Groundbreaking Study!
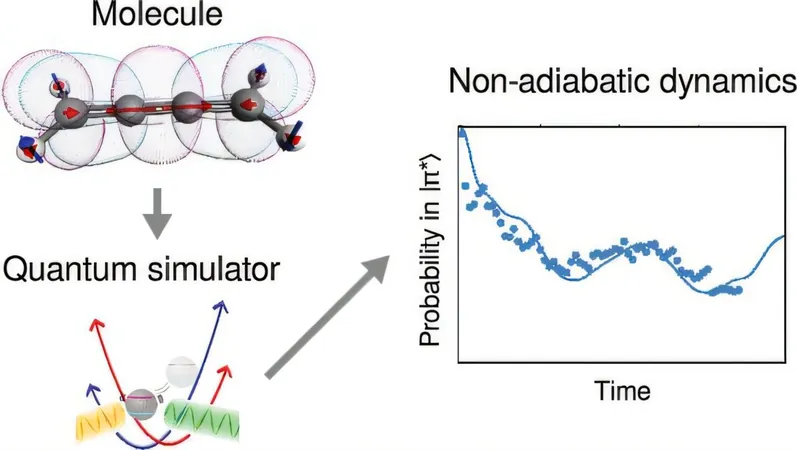
In a game-changing study published in the Journal of the American Chemical Society, researchers have demonstrated a significant leap forward: not just measuring molecules, but simulating how they evolve over time.
How Did They Do It?
Using a trapped-ion quantum computer, this innovative approach utilizes manipulation of individual atoms in a vacuum, stabilized by electromagnetic fields. Instead of relying solely on traditional quantum bits (qubits), the researchers cleverly incorporated vibrations of the atoms in their quantum system, known as "bosonic modes." This mixed qudit-boson simulation is a revolutionary technique that dramatically shrinks the size of quantum computers needed for molecule simulations.
Real Molecules in the Spotlight!
The study showcased simulations of three important molecules—allene, butatriene, and pyrazine—each showcasing intricate electronic and vibrational interactions after light absorption. By employing a laser and a single ion, the team managed to slow down these rapid processes by an astonishing factor of 100 billion, allowing them to visualize these interactions in real-time. While these reactions occur in femtoseconds in reality, their simulation lasted milliseconds—an eternity in quantum terms, letting researchers observe and study the reactions.
Efficiency Beyond Compare!
What sets this breakthrough apart is its remarkable efficiency. Traditional quantum simulations that sought to replicate these processes would require upwards of 11 qubits and around 300,000 error-free entangling operations—a monumental challenge for current technology. In stark contrast, this new method executed the same operations using just a single trapped ion and a laser pulse, boasting an efficiency improvement estimated at over a million times!
Turning Chaos into Clarity!
Moreover, the researchers tackled the challenging concept of "open-system" dynamics, where molecules interact with their surrounding environment—a task notoriously difficult for classical computers. By strategically introducing controlled noise into the simulation, they were able to mimic how real molecules dissipate energy, proving that even the messiness of real-world interactions can be effectively captured through quantum simulations.
The Future of Quantum Chemistry!
This pioneering work marks a significant milestone for quantum chemistry. Even though today’s quantum computers are still in their infancy, the methods pioneered in this research indicate that small-scale experiments can solve scientifically relevant problems.
With just a modest increase in scale—potentially reaching 20 or 30 ions—these quantum simulations could tackle chemical systems far beyond the capabilities of today’s best classical supercomputers. This opens wide the doors to groundbreaking advancements in drug discovery, sustainable energy solutions, and a deeper understanding of the fundamental chemical processes that sustain life.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)