Revolutionizing Catalysis: Groundbreaking Porous Thin-Film Technology Boosts Reaction Efficiency
2025-03-10
Author: Arjun
Introduction
Catalysis plays a crucial role in various industrial reactions, particularly in the large-scale production of drugs, polymers, and other critical compounds. To improve the efficiency and effectiveness of these processes, ongoing advancements in catalytic methods are essential.
Current catalysts can be classified into homogeneous and heterogeneous types. Homogeneous catalysts, while effective, share the same phase as the reactants and can be challenging to remove from mixtures. Conversely, heterogeneous catalysts are often favored due to their ease of separation and reusability, making them significantly more practical for industrial applications.
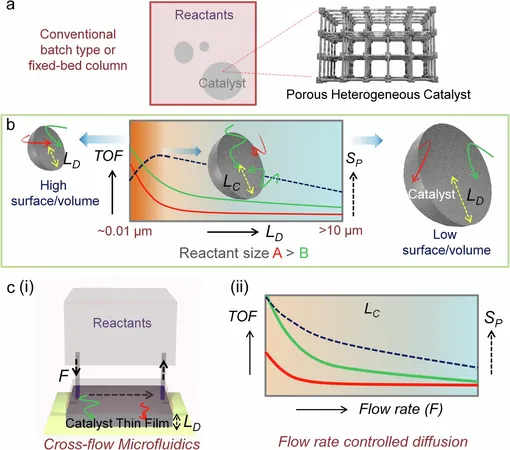
Emergence of Porous Heterogeneous Catalysts
In recent years, the emergence of porous heterogeneous catalysts has marked a significant advancement in this field. These catalysts provide enhanced opportunities for reusability and increase the density of their catalytic sites, thereby improving performance.
A noteworthy advancement comes from Ritesh Haldar’s research group at TIFR Hyderabad, whose latest work, published in *Nature Communications*, introduces a pioneering methodology to further magnify the effectiveness of porous heterogeneous catalysts.
Innovative Cross-Flow Microfluidic System
The team has developed a novel cross-flow microfluidic system that incorporates a porous heterogeneous thin film. This innovative design immobilizes the catalyst on a solid substrate, allowing the reactants to interact efficiently with the catalytic surface without the risk of losing the catalyst in the process.
The microfluidic setup operates by pushing reactants through the inlet and directing the products back through the outlet. Remarkably, if the initial conversion rate of reactants to products is 25%, this system can enable multiple consecutive cycles, leading to significantly higher overall reaction efficiency.
Breakthrough in Catalytic Performance
One of the breakthrough aspects of this research is the introduction of a new method to assess the effectiveness of the integrated cross-flow microfluidic system. The team successfully conducted a base-catalyzed Knoevenagel condensation reaction, achieving an impressive turnover frequency (TOF) exceeding 4000 h⁻¹.
TOF is a critical measure indicating how effectively a catalyst converts reactants into products relative to its amount over time; the dramatic increase in TOF observed in this study highlights the enhanced diffusion rates of reactants and the optimized immobilization of the catalyst.
Future Directions
However, it is important to note that the current findings are limited to thin films of catalysts and liquid-phase organic reactions. The research team aims to broaden their exploration to include gas-phase reactions and large-scale chemical processes, leveraging their innovative cross-flow microfluidic technology.
This next stage of development could revolutionize various organic reaction applications, potentially transforming industrial catalysis as we know it.
Conclusion
With the promise of increased efficiency and effectiveness, this groundbreaking advancement in catalytic technology stands to not only reduce production costs but also minimize environmental impacts, paving the way for a more sustainable future in chemical manufacturing.
Stay tuned for more updates on this game-changing research!
 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)